13.3
Impact Factor
Theranostics 2019; 9(1):90-103. doi:10.7150/thno.30259 This issue Cite
Research Paper
Redox Dual-Responsive and O2‑Evolving Theranostic Nanosystem for Highly Selective Chemotherapy against Hypoxic Tumors
1. State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, China.
2. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
* Contributed equally to this work.
Abstract
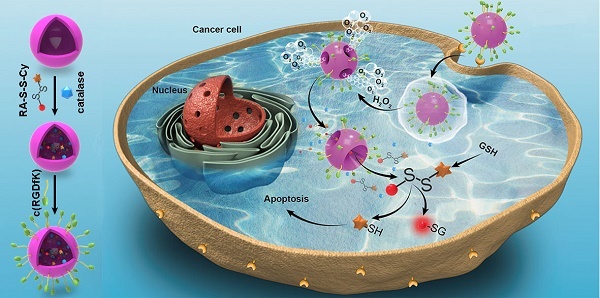
Activatable theranostic agents, which combine fluorescent reporters with masked chemotherapeutic agents that are activated by tumor-associated stimuli, would be attractive candidates to improve the tumor selectivity of chemotherapy. This work reports a ROS/GSH dual-activatable and O2‑evolving theranostic nanosystem (RA-S-S-Cy@PLGA NPs) for highly selective therapy against hypoxic tumors and in situ fluorescence-tracking of cancer chemotherapy.
Methods: In this system, the newly designed theranostic agent (RA-S-S-Cy) is composed of a disulfide bond as a cleavable linker, a near infrared (NIR) active fluorophore as a fluorescent tracker, and a natural cyclopeptide RA-V as the active anti-cancer agent. Upon reaction with the high level of intracellular glutathione (GSH), disulfide cleavage occurs, resulting in concomitant active drug RA-V release and significant NIR fluorescence increase. To further improve the tumor targeting of RA-S-S-Cy and achieve redox dual-responsiveness, RA-S-S-Cy was incorporated into the c(RGDfK)-targeted PLGA nanoparticles together with an O2-generating agent (catalase) to produce RA-S-S-Cy@PLGA NPs.
Results: The cell-specific and redox dual-activatable release of RA-V lead to enhanced therapeutic outcomes in vivo and in vitro. More significantly, the RA-S-S-Cy@PLGA NPs were successfully applied for monitoring of drug release and chemotherapeutic efficacy in situ by “turn-on” NIR fluorescence.
Conclusions: RA-S-S-Cy@PLGA NPs would be efficient theranostic nanosystems for more precise therapy against hypoxic tumors and provides a potential tool for deeper understanding of drug release mechanisms.
Keywords: cancer, theranostic system, hypoxic tumor, ROS/GSH dual-activatable, cyclopeptide RA-V, fluorescence imaging
 Global reach, higher impact
Global reach, higher impact