13.3
Impact Factor
Theranostics 2018; 8(16):4509-4519. doi:10.7150/thno.27428 This issue Cite
Research Paper
CD28null pro-atherogenic CD4 T-cells explain the link between CMV infection and an increased risk of cardiovascular death
1. Department of Clinical and Experimental Medicine, Brighton and Sussex Medical School, Brighton, United Kingdom.
2. Immunology and allergy GC-01 group Maimonides Biomedicine Institute of Cordoba (IMIBIC), Reina Sofia Hospital, University of Cordoba, Cordoba, Spain.
3. Department of Global Health and Infectious Diseases, Brighton and Sussex Medical School, Brighton, United Kingdom.
4. School of Pharmacy and Biomolecular Sciences, University of Brighton.
5. Family Medicine and Primary Care, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore.
6. School of Engineering and Informatics, University of Sussex, Brighton, UK.
7. University of Malawi, The Polytechnic, Blantyre, Malawi.
8. Institute for Transfusion Medicine, Hannover Medical School, Hannover, Germany.
†School of Life Sciences University of Hull, Hull, UK.
Abstract
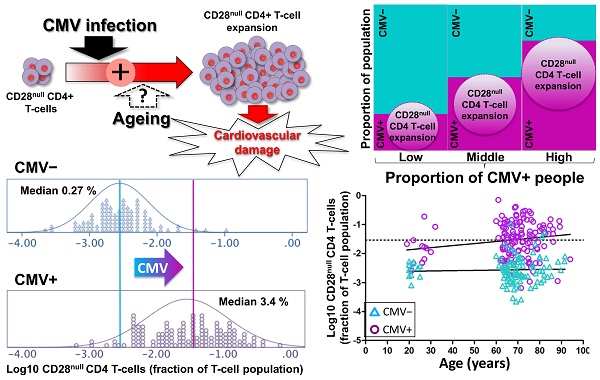
An increased risk of cardiovascular death in Cytomegalovirus (CMV)-infected individuals remains unexplained, although it might partly result from the fact that CMV infection is closely associated with the accumulation of CD28null T-cells, in particular CD28null CD4 T-cells. These cells can directly damage endothelium and precipitate cardiovascular events. However, the current paradigm holds that the accumulation of CD28null T-cells is a normal consequence of aging, whereas the link between these T-cell populations and CMV infection is explained by the increased prevalence of this infection in older people. Resolving whether CMV infection or aging triggers CD28null T-cell expansions is of critical importance because, unlike aging, CMV infection can be treated.
Methods: We used multi-color flow-cytometry, antigen-specific activation assays, and HLA-typing to dissect the contributions of CMV infection and aging to the accumulation of CD28null CD4 and CD8 T-cells in CMV+ and CMV- individuals aged 19 to 94 years. Linear/logistic regression was used to test the effect of sex, age, CMV infection, and HLA-type on CD28null T-cell frequencies.
Results: The median frequencies of CD28null CD4 T-cells and CD28null CD8 T-cells were >12-fold (p=0.000) but only approximately 2-fold higher (p=0.000), respectively, in CMV+ (n=136) compared with CMV- individuals (n=106). The effect of CMV infection on these T-cell subsets was confirmed by linear regression. Unexpectedly, aging contributed only marginally to an increase in CD28null T-cell frequencies, and only in CMV+ individuals. Interestingly, the presence of HLA-DRB1*0301 led to an approximately 9-fold reduction of the risk of having CD28null CD4 T-cell expansions (OR=0.108, p=0.003). Over 75% of CMV-reactive CD4 T-cells were CD28null.
Conclusion: CMV infection and HLA type are major risk factors for CD28null CD4 T-cell-associated cardiovascular pathology. Increased numbers of CD28null CD8 T-cells are also associated with CMV infection, but to a lesser extent. Aging, however, makes only a negligible contribution to the expansion of these T-cell subsets, and only in the presence of CMV infection. Our results open up new avenues for risk assessment, prevention, and treatment.
Keywords: CD28null CD4 T-cells, CD28null CD8 T-cells, cardiovascular disease, aging, Cytomegalovirus, coronary complications
 Global reach, higher impact
Global reach, higher impact