13.3
Impact Factor
Theranostics 2018; 8(13):3584-3596. doi:10.7150/thno.25409 This issue Cite
Research Paper
Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose
1. Guangdong Key Laboratory of Nanomedicine, Shenzhen engineering Laboratory of nanomedicine and nanoformulations, CAS Key Lab for Health Informatics, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, P. R. China
2. Department of Chemistry, Guangdong Medical University, Dongguan 523808, PR China
3. University of Chinese Academy of Sciences, Beijing 100049, PR China
‡ These authors contributed equally to this work.
Abstract
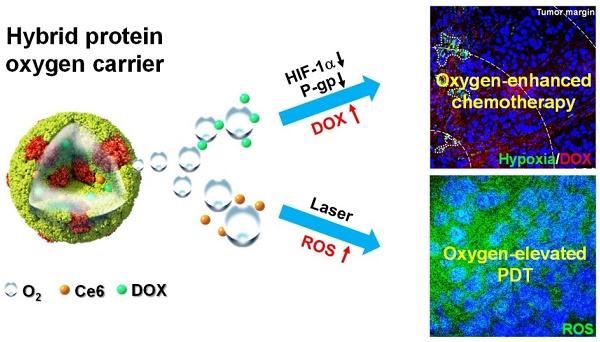
Hypoxia is a characteristic feature of solid tumors and an important causation of resistance to chemotherapy and photodynamic therapy (PDT). It is challenging to develop efficient functional nanomaterials for tumor oxygenation and therapeutic applications.
Methods: Through disulfide reconfiguration to hybridize hemoglobin and albumin, tumor-targeted hybrid protein oxygen carriers (HPOCs) were fabricated, serving as nanomedicines for precise tumor oxygenation and simultaneous enhancement of hypoxia-dampened chemotherapy and photodynamic therapy. Based on encapsulation of doxorubicin (DOX) and chlorin e6 (Ce6) into HPOCs to form ODC-HPOCs, the mechanism and therapeutic efficacy of oxygen-enhanced chemo-PDT was investigated in vitro and in vivo.
Results: The precise oxygen preservation and release of the HPOC guaranteed sufficient tumor oxygenation, which is able to break hypoxia-induced chemoresistance by downregulating the expressions of hypoxia-inducible factor-1α (HIF-1α), multidrug resistance 1 (MDR1) and P-glycoprotein (P-gp), resulting in minimized cellular efflux of chemodrug. Moreover, the oxygen supply is fully exploited for upgrading the generation of reactive oxygen species (ROS) during the photodynamic process. As a result, only a single-dose treatment of the HPOCs-based chemo-PDT exhibited superior tumor suppression. The combination therapy was guided by in vivo fluorescence/photoacoustic imaging with nanoparticle tracking and oxygen monitoring.
Conclusion: This well-defined HPOC as a versatile nanosystem is expected to pave a new way for breaking multiple hypoxia-induced therapeutic resistances to achieve highly effective treatment of solid tumors.
Keywords: hybrid protein nanoparticle, targeted oxygen carrier, tumor hypoxia, chemotherapy, photodynamic therapy
 Global reach, higher impact
Global reach, higher impact