13.3
Impact Factor
Theranostics 2018; 8(4):1159-1167. doi:10.7150/thno.23373 This issue Cite
Research Paper
Delivery of Sonic Hedgehog Gene Repressed Irradiation-induced Cellular Senescence in Salivary Glands by Promoting DNA Repair and Reducing Oxidative Stress
1. Institute for Regenerative Medicine, Molecular and Cellular Medicine Department, College of Medicine, Texas A&M University Health Science Center, College Station, Texas 77843, USA.
2. Department of Small Animal Clinical Sciences, College of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, Texas 77843, USA.
Received 2017-10-17; Accepted 2017-12-1; Published 2018-1-13
Abstract
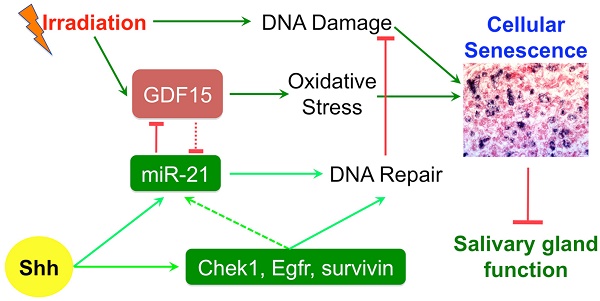
Rationale: Irreversible hypofunction of salivary glands or xerostomia is common in head and neck cancer survivors treated with radiotherapy even when various new techniques are applied to minimize the irradiation (IR) damage. This condition severely impairs the quality of life of patients and can only be temporarily relieved with current treatments. We found recently that transient expression of Sonic Hedgehog (Shh) in salivary glands after IR rescued salivary function, but the underlying mechanisms are not totally clear.
Methods: We generated a mouse model of IR-induced hyposalivation, and delivered adenoviral vectors carrying Shh or control GFP gene into submandibular glands (SMGs) via retrograde ductal instillation 3 days after IR. The cellular senescence was evaluated by senescence-associated beta-galactosidase assay and the expression of senescence markers. The underlying mechanisms were explored by examining DNA damage, oxidative stress, and the expression of related genes by qRT-PCR, Western blot and immunofluorescent staining.
Results: Shh gene transfer repressed IR-induced cellular senescence by promoting DNA repair and decreasing oxidative stress, which is mediated through upregulating expression of genes related to DNA repair such as survivin and miR-21 and repressing expression of pro-senescence gene Gdf15 likely downstream of miR-21.
Conclusion: Repressing cellular senescence contributes to the rescue of IR-induced hyposalivation by transient activation of Hh signaling, which is related to enhanced DNA repair and decreased oxidative stress in SMGs.
Keywords: Irradiation-induced hyposalivation, cellular senescence, Hedgehog signaling, DNA repair, oxidative stress
Introduction
Head and neck cancer (HNC) is the fifth most common cancer with 64,970 estimated new cases in 2015 [1] and about 342,550 estimated survivors in 2012 in USA [2]. Radiotherapy is a common form of treatment for HNC, and nondiseased salivary glands (SGs) are often exposed to irradiation (IR). Due to the exquisite radiosensitivity of salivary glands, irreversible hyposalivation or xerostomia is very common (>60%) in long-term HNC survivors treated with conventional radiotherapy [3]. Novel intensity-modulated radiotherapy significantly decreased IR dose to salivary glands and the incidence of xerostomia, but about 20% of patients treated with such novel radiotherapies still have long-term xerostomia and related weight loss [4]. Hyposalivation exacerbates dental caries and periodontal disease, and causes problems of mastication, swallowing, speech and sleep, a burning sensation of the mouth, and loss of taste, which severely impairs the quality of life of patients. The irreversible hyposalivation is caused by loss or impairment of saliva-producing acinar cells and their replacement by connective tissue and fibrosis, which has been attributed to the loss of functional glandular stem/progenitor cells [5], the impairment of parasympathetic innervation [6], and the damage of microvessels [7]. Current treatments for IR-induced xerostomia, such as artificial saliva and saliva secretion stimulators, can only temporarily relieve these symptoms.
Hedgehog (Hh) intercellular signaling pathway trigged by Hh ligands is highly conserved during evolution, required for salivary gland branching morphogenesis [8], and activated during the functional regeneration of adult salivary glands after occlusion damages [9]. We found recently that the transient expression of Hh ligand Sonic Hedgehog (Shh) transgene in SGs after IR rescued IR-damaged salivary gland function, which is related to the preservation of putative stem/progenitor cells, parasympathetic innervation and microvessels following transient activation of Hh signaling [10, 11]. The cellular senescence is a newly identified mechanism driving the IR-induced loss of salivary function [12]. We report in this study that the Shh gene transfer following IR inhibited the IR-induced cellular senescence through promoting DNA damage repair and decreasing oxidative stress, which is mediated by the induction of anti-senescence factors such as survivin and miR-21 and the consequent repression of miR-21 targeted pro-senescence factor GDF15. These data revealed novel mechanisms of IR-induced hyposalivation and Hh-mediated rescue, which will help to optimize strategies for preserving or restoring salivary gland function following radiotherapy in HNC patients.
Materials and Methods
The mouse model of IR-induced hyposalivation
8-10-week-old C57BL/6 mice were purchased form the Jackson Laboratory (Bar Harbor, ME, USA). In each experiment, at least 5 animals are used for each group. All animal procedures were performed under a protocol approved by Texas A&M University Health Science Center IACUC committee. Since we did not observe significant gender differences in the activation of Hh signaling and the rescue of IR-induced hyposalivation by Shh gene transfer in the same mouse model [11], we only used male mice in the current study. Mice were anesthetized with Xylazine/Ketamine mixture (4 mg/mL Xylazine mixed with 30 mg/mL Ketamine, 4 mL/kg, intraperitoneally), and the head-and-neck region was exposed to 15 Gy of 6 MeV electron beam produced by a medical linear accelerator (Clinac 2100C, Varian Medical Systems, Palo Alto, California) as reported previously [11].
Preparation and delivery of adenoviral vectors into SMGs
Adenoviral vector encoding green fluorescent protein (GFP) or rat Shh (Ad-GFP or Ad-Shh; Applied Biological Materials, Canada) was expanded in 293A cells and purified by two rounds of ultracentrifugation through a cesium chloride gradient. The titers (particles/mL) of purified vectors were determined by qPCR with an Adeno-X rapid titer kit (Clontech Laboratories, Mountain View, CA). For gene delivery, mice were anesthetized with Xylazine/Ketamine as mentioned above, and vectors were delivered to both SMGs by retrograde ductal instillation as we reported previously [11] at a dose of 1 x 109 particles per SMG on day 3 after IR.
Quantitative RT-PCR
Reagents and instrument for quantitative reverse transcription polymerase chain reaction (qRT-PCR) were as reported previously [10]. Primers for mRNAs were synthesized by Invitrogen with sequences retrieved from Primerbank (http://pga.mgh.harvard.edu/primerbank) and using Gapdh as the reference RNA. qRT-PCR analyses for microRNAs were performed with TaqMan miRNA assays (Thermo Fisher, USA) using U6 snRNA as the reference RNA. PCR assays were run in triplicates with 3 independent samples of each group and analyzed with qBasePlus software (Biogazelle, Belgium).
Detection of SA-β-gal on SMG sections
Detection of SA-β-gal activity was performed at pH 5.5 as reported [12]. Briefly, frozen sections of SMG were fixed with 0.5% gluteraldehyde in PBS for 15 min, washed with PBS supplemented with 1 mM MgCl2, and stained for 5-6 h in PBS containing 1 mM MgCl2, 1 mg/mL X-Gal, and 5 mM of each potassium ferricyanide and potassium ferrocyanide and counterstained with Nuclear Fast Red (Vector Labs). The percentages of area positive for SA-β-gal staining were quantified with NIS-Elements AR software (Nikon) from 6 representative fields of 3 independent samples per group.
Western blot and immunofluorescence staining analyses
For Western blot, fresh SMG samples were homogenized with tissue protein extraction reagent (T-PER, 40 μL per mg tissue) containing protease inhibitors (Thermo Fisher, USA) followed by centrifugation at 10,000 × g for 5 min to collect supernatant. Western blot was performed as reported [10] with antibodies for Aqp5, survivin, GDF15 and Gapdh (Abcam, USA, diluted 1:1000). The intensities of Western blot signals for all detected proteins were quantified with Quantity One software (Bio-Rad) and normalized to that of Gapdh. For immunofluorescence (IF) staining, frozen SMG sections were first incubated in a 1:100 dilution of antibodies for γH2AX (Millipore), survivin or GDF15, washed and incubated with appropriate secondary antibodies labeled with Texas red (Vector Laboratories, USA), and counterstained nuclei with 4',6-diamidino-2-phenylindole (DAPI).
Measurement of ROS, MDA and SOD
Intracellular ROS levels were quantified by OxiSelect™ In Vitro ROS/RNS Assay kit (Cell Biolabs, USA) per the instructions of the vendor. Briefly, homogenized SMGs were incubated with 10 μM 2, 7-dichlorofluorescein diacetate (DCF-DA) for 1 h at 37 °C in the dark, and then the luminescent product 2,7-dichlorodihydro-fluorescein diacetate (H2DCF-DA) produced in proportion to ROS levels was measured by a spectrometer. The SOD activities and MDA level in SMGs were analyzed using a SOD activity assay kit (Abcam, ab65354) and a Lipid Peroxidation Assay kit (Abcam, ab118970) according to the manufacturer's instructions.
In vitro assays on HSG cells
Human submandibular gland intercalated duct cell line HSG [13] is a kind gift from Dr. Changyu Zheng in NIH. HSG cells were cultured in Dulbecco's modified Eagle's medium/Ham's F-12 (1:1) containing 5% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin (Life Technologies). For establishment of HSG cells expressing lower levels of GDF15, HSG cells were transduced with lentiviral vectors carrying GFP and shRNAs against human GDF15 or scrambled shRNAs (Applied Biological Materials) at a multiplicity of infection (MOI) of 10, and GFP+ cells were sorted by FACS for expansion. 50-60% confluent HSG cells on 6-well plates were irradiated with RS2000 irradiator (Radsource) at 4 Gy. Some parent HSG cells were treated with 200 ng/mL recombinant human GDF15 (Abcam) or bovine serum albumin (BSA) for 48 h. SA-β-gal activity in HSG cells was examined as mentioned for SMG sections, and the percentages of cells positive for SA-β-gal staining were counted in 6 representative fields per treatment. MDA levels in HSG cells were examined as mentioned for SMG tissues using 2 x 106 cells per sample.
Statistics
All quantified data were analyzed using one-way ANOVA followed by Tukey multiple-comparison test. Statistical analysis and graphical generation of data were done with GraphPad Prism software (San Diego, CA).
Results
Transient activation of Hh signaling repressed IR-induced cellular senescence
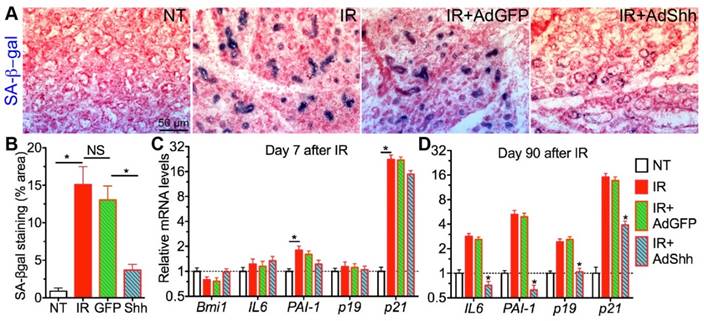
To determine whether transient Hh activation following IR affects IR-induced cellular senescence in salivary gland, we collected SMG samples 7 or 90 days after IR with or without transfer of GFP or Shh genes on day 3, and examined the expression of senescence markers in SMGs including senescence-associated β-galactosidase (SA-β-gal), p19INK4d and p21Waf1 and senescence-associated secretory phenotype (SASP) factors PAI-1 and IL6 [12]. On day 7, IR significantly upregulated the activity of SA-β-gal and the expression of p21Waf1 and PAI-1 but not that of p19INK4d and IL6 compared to the non-treated (NT) control group; whereas, the delivery of Ad-Shh but not Ad-GFP three days after IR dramatically repressed the increases of SA-β-gal activity (Fig. 1A,B, P<0.001) but only slightly repressed the upregulation of PAI-1 and p21Waf1 expression (Fig. 1C, P>0.05). Moreover, the expression of Bmi1 that mediated repression of p21Waf1 expression after Hh activation in other tissues [14, 15] was not significantly affected by IR with or without Ad-Shh treatment on day 7 (Fig. 1C, P>0.05). On day 90, IR significantly upregulated the expression of IL6, PAI-1, p19INK4d and p21Waf1 in SMGs (Fig. 1D). Although the adenovirus-mediated Shh gene transfer only activated Hh signaling in SMGs for less than 10 days [11], such a transient Hh activation significantly repressed the upregulation of all examined senescence markers on day 90 (Fig. 1D). These data indicated that transient Hh activation in SMGs repressed IR-induced cellular senescence both short-term and long-term, which did not appear to be through acute repression of cell cycle inhibitors and SASP factors IL6 and PAI-1.
Short-term and long-term effects of Shh gene transfer on IR-induced cellular senescence in SMGs. A-B: SA-βgal staining of SMG sections 7 days after IR and the quantification of SA-βgal+ area (N=6). C-D: qRT-PCR analysis on mRNA expression of senescence marker genes 7 or 90 days after IR with or without intragland delivery of GFP or Shh gene. N = 3. *: P < 0.05 vs. IR unless labeled otherwise.
Transient Hh activation promoted the repair of IR-induced DNA damages by upregulating multiple related genes
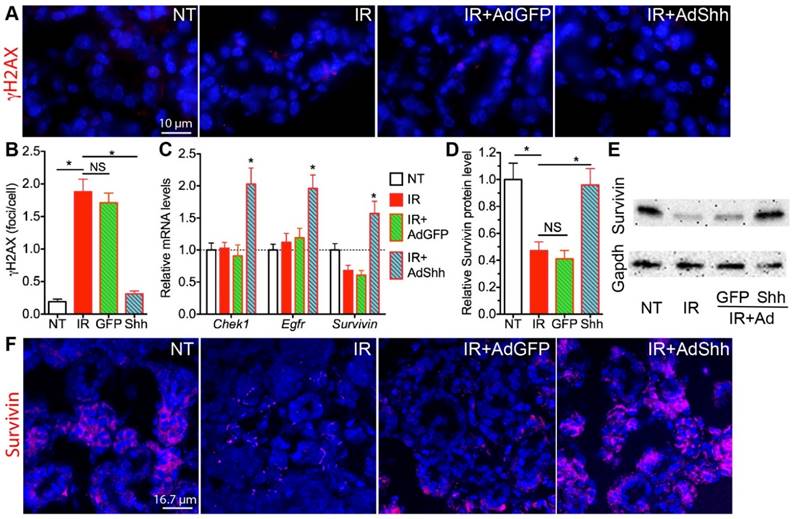
The persistent DNA double strand breaks (DSB) damage, visualized by phosphorylated H2AX (γH2AX) foci in the nucleus detectable between hours to weeks after IR, is a hallmark and a major cause of cellular senescence in irradiated salivary glands [12]. In SMG samples collected 7 days after IR, γH2AX foci numbers were significantly increased in the IR alone group as expected, and this increase was significantly repressed by the delivery of Ad-Shh but not Ad-GFP three days after IR (Fig. 2A,B). In various cancer cells, Hh signaling pathway regulates the expression of multiple genes related to repair of IR-induced DNA damages, including Bid, Chek1, Egfr, FEN1, survivin, PRKDC/DNA-PKcs, XRCC4, and XRCC6 [16-20]. We examined the mRNA levels of these genes in irradiated SMGs by qRT-PCR and found that mRNA levels of Chek1, Egfr and survivin were significantly upregulated by the transfer of Shh but not GFP gene compared to IR only samples (Fig. 2C), whereas the other 5 genes mentioned above were not significantly affected (data not shown). Among these three upregulated genes, only survivin mRNA level was significantly downregulated by IR compared to NT and survivin is a known direct target gene of Hh signaling [21], so we further examined the protein level of survivin in SMGs by IF and Western blot, and confirmed that transient activation of Hh signaling pathway significantly restored expression of survivin protein in SMGs downregulated by IR (Fig. 2D-F). Chek1 promotes multiple DNA repair pathways [22], Egfr facilitates DNA synthesis and repair through modification of Histone H4 [23], and survivin enhances the kinase activity of DNA-PKcs required for the non-homologous end joining (NHEJ) pathway of DNA repair [20]. The restoration and upregulation of these three genes likely all contributed to the enhanced repair of DNA damage after transient Hh activation.
Transient Hh activation reduced oxidative stress caused by IR through inhibition of GDF15 upregulation
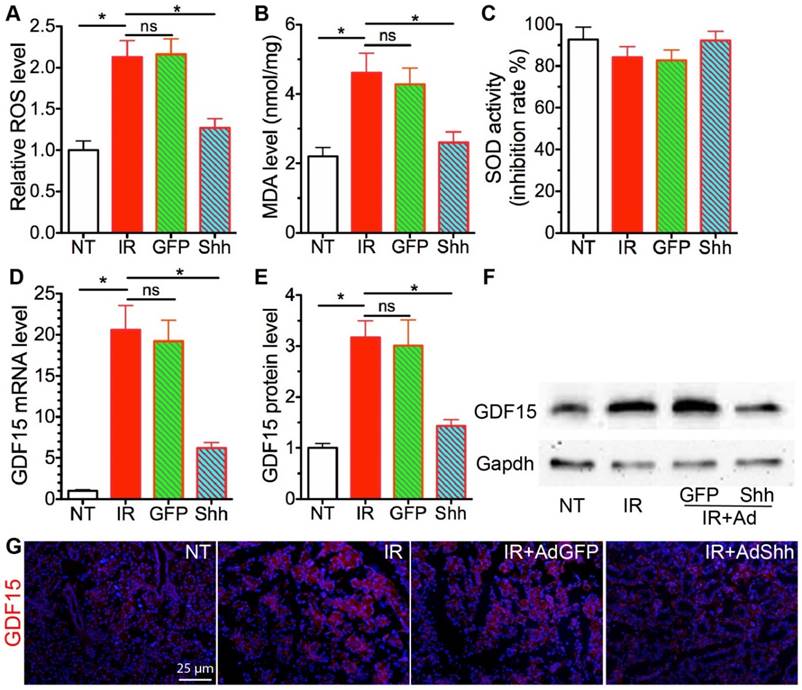
IR enhances production of reactive oxygen species (ROS) that induce oxidative stress in salivary glands [24]. ROS is involved in both the induction and stabilisation of cellular senescence: ROS can accelerate telomere shortening and damage DNA directly, and thus induce senescence [25, 26]; on the other hand, characteristics of cellular senescence such as mitochondrial dysfunction and p21 upregulation lead to increased ROS generation that may help stabilize the senescence [27]. Hh activation significantly reduced ROS level in astrocytes by increasing levels of the antioxidative proteins catalase (Cat) and superoxide dismutase (Sod) [28]. Hence, we examined the level of ROS and the marker of oxidative stress malondialdehyde (MDA) in our SMG samples. IR significiantly increased the level of ROS and MDA in SMGs as expected, whereas transient Hh activation by transfer of Shh gene but not control GFP gene significantly inhibited the increase of ROS and MDA (Fig. 3A,B). But, we did not observe significant changes in Sod activities or mRNA expression of Cat and Sod1/2/3 in irradiated SMGs after Shh gene transfer (Fig. 3C and data not shown). Growth differentiation factor-15 (GDF15) is upregulated by IR in various types of tissues and cells [29, 30], is essential for ROS generation in irradiated human endothelial cells and their consequent senescence [31], and is a potential Hh target gene upregulated by overexpression of Gli3 that functions as a repressor of Hh signaling in embryonic development [32]. In irradiated SMGs, the expression of GDF15 increased dramatically at both mRNA and protein levels compared to non-treated SMGs, transient Hh activation inhibited upregulation of GDF15 by IR as indicated by qRT-PCR, Western blot and IF (Fig. 3D-G). Cellular senescence induced by GDF15 in human endothelial cells is through a p16INK4a/Rb-dependent pathway, whereas the expression of p16 and Rb1 in mouse SMGs collected on day 7 after IR was not significantly affected by IR with or without Ad-Shh treatment (data not shown).
GDF15 upregulation is essential for IR-induced oxidative stress and senescence in SG epithelial cells
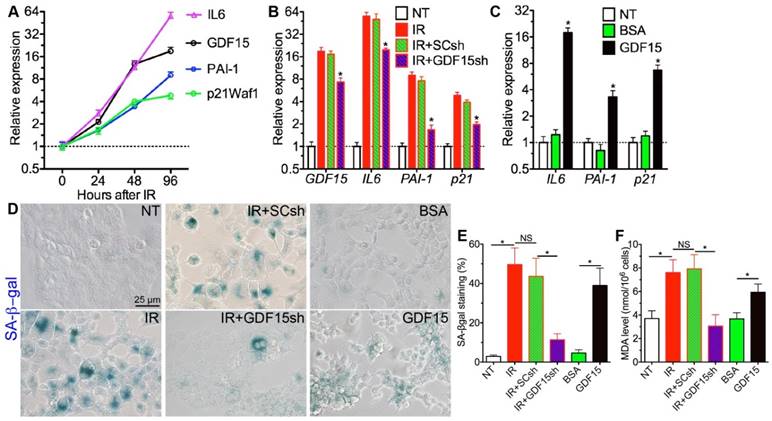
To determine whether GDF15 is essential for IR-induced senescence in SGs, we used human submandibular gland intercalated duct cell line HSG [13] as the in vitro model. From 24 h to 96 h after IR, GDF15 mRNA level increased significantly and gradually in HSG cells, and the expression of senescence markers IL6, PAI1 and p21 also increased in a similar manner (Fig. 4A), whereas the expression of p16 and Rb1 was not significantly affected by IR at any time point examined (data not shown). We then knocked down the expression of GDF15 in HSG cells immediately before IR, and found that the upregulation of GDF15 and all senescence markers by IR at 96 h was significantly repressed by shRNAs against GDF15 (GDF15sh) but not by scrambled shRNAs (SCsh) (Fig. 4B). Conversely, treatment with human recombinant GDF15 protein for 48 h significantly increased the mRNA expression of senescence markers IL6, PAI-1 and p21 in HSG cells compared to either non-treated (NT) or BSA-treated cells (Fig. 4C). Consistent with the qRT-PCR data, the SA-βgal activity upregulated by IR in HSG cells was repressed by GDF15sh but not SCsh, whereas recombinant GDF15 protein but not BSA treatment increased SA-β-gal activity (Fig. 4D,E). IR increased MDA level in HSG cells as expected, whereas GDF15 knockdown significantly inhibited the upregulation of MDA by IR (Fig. 4F). Consistently, GDF15 protein but not BSA treatment significantly increased the MDA level in HSG cells (Fig. 4F). These data collectively indicated that GDF15 is a key mediator of IR-induced oxidative stress and senescence in SG epithelial cells.
Acute effects of transient Hh activation on DNA damage repair and expression of related genes. A,B: Shh gene transfer after IR decreased numbers of γH2AX+ DNA damage foci. C: Shh gene transfer induced mRNA expression of genes related to DSB repair including Egfr, Chek1 and Survivin. D,E: Western blot analysis of Survivin expression in SMGs. F: Immunofluorescent staining of Survivin in SMG sections. All samples were collected 7 days after IR. N = 3. *: P < 0.05 vs. IR. NS: not significant, P > 0.05.
Acute effects of transient Hh activation on IR-induced oxidative stress and GDF15 expression. A-B: The levels of ROS and MDA in SMGs were analyzed with ROS/RNS Assay kit and Lipid Peroxidation Assay kit and calibrated with standard curves for H2O2 or MDA respectively. C: The SOD activities in SMGs showed as inhibition rate of xanthine oxidase activity. D-G: The expression of GDF15 in SMGs was analyzed with qRT-PCR (D), Western blot (E,F), and immunofluorescent staining (G). N = 3. *: P < 0.05 vs. IR. NS: not significant vs. IR.
Transient Hh activation induced miR-21 that inhibits GDF15 expression and promotes DNA repair
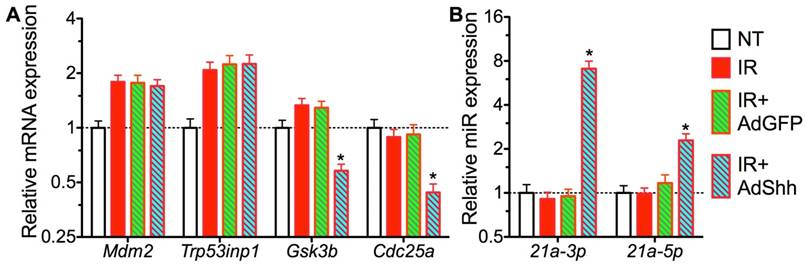
How Hh signaling regulates GDF15 expression is unclear. The expression of GDF15 is regulated by p53 pathway [33], and Trp53inp1 was required for IR-induced maximal elevation of GDF15 expression in human fibroblasts [34]. Since transient Hh activation did not repress the upregulation of Trp53inp1 and other p53 target genes including p21 and Mdm2 by IR on day 7 (Fig. 1B and 5A), we speculated that the repression of GDF15 upregulation by Hh activation is through epigenetic mechanisms. GDF15 mRNA contains a binding site for miR-21 [35], and miR-21 expression is positively regulated by Hh signaling [36], hence we examined miR-21 expression in our SMG samples collected 7 days after IR by qRT-PCR. We found that, compared to NT, the expression of miR-21a-5p and -3p was not significantly affected by IR alone or IR+Ad-GFP, but was dramatically upregulated by Ad-Shh treatment following IR (Fig. 5B). Moreover, both miR-21a-5p and -3p were reported recently to promote DNA DSB repair caused by IR in tumor cells through NHEJ and homologous recombination repair (HRR) by targeting GSK3b and Cdc25a [37]. In irradiated SMGs, we confirmed that transfer of Shh gene but not control GFP gene significantly decreased the mRNA expression of GSK3b and Cdc25a compared to IR alone or the IR+Ad-GFP group (Fig. 5A), suggesting that miR-21 also contributes to the enhanced DNA DSB repair following transient Hh activation.
GDF15 is essential for senescence in irradiated salivary gland epithelial cells. A: Expression of senescence-related genes in HSG cells after 4 Gy IR. B: Effects of GDF15 knockdown on the upregulation of senescence-related genes in HSG cells by IR, N = 3, *: P < 0.05 vs. IR. C: Effects of recombinant GDF15 protein on the expression of senescence-related genes in HSG cells, N = 3, *: P < 0.05 vs. NT or BSA. D-F: Effects of IR, IR+GDF15 knockdown, and GDF15 protein on SA-β-gal activities (D-E) and MDA levels in HSG cells (F). All samples in B-F were collected 96 h after IR or 48 h after protein treatment.
Mechanisms of GDF15 inhibition by activation of Hh signaling. A: Expression of target genes of p53 pathway and miR-21 related to GFD15 regulation and DNA DSB repair respectively. B: Expression of miR-21 targeting GDF15. N = 3. *: P < 0.05 vs. IR or IR+Ad-GFP.
Discussion
We found recently that transient Shh expression shortly after IR rescued SG function through the preservation of putative stem/progenitor cells, parasympathetic innervation and microvessels in mouse models, and saliva secretion and expression of acinar markers were preserved for up to 120 days after IR [10, 11]. Here we report that Shh gene transfer significantly repressed IR-induced cellular senescence both short-term and long-term, which also contributed to the rescue of salivary gland function following IR. IR induces cellular senescence in SGs manifested by the expression of senescence-associated genes including cell cycle inhibitors and SASP factors such as IL6 and PAI-1, whereas the depletion of one key SASP factor IL6 significantly repressed the IR-induced senescence and hyposalivation, indicating that cellular senescence is a fundamental mechanism driving IR-induced damages in SGs [12]. However, the upregulation of SASP factors IL6 and PAI-1 and cell cycle inhibitors p19 and p21 by IR was only efficiently repressed long but not shortly after Shh gene transfer, suggesting that Hh activation repress IR-induced senescence through other mechanisms.
Persistent DNA damage and oxidative stress are essential for IR-induced senescence. We found that transient Hh activation decreased both DNA damage and oxidative stress in irradiated SMGs 4 days after gene transfer. The decreased DNA damage is related to the upregulation of multiple genes contributing to DNA DSB repair including Chek1, Egfr, survivin, and miR-21. When activated in response to DNA damage, oxidative stress and replication stress, Chek1 promotes multiple DNA repair pathways by phosphorylating DNA repair inhibitors such as Cdc25a to facilitate their degradation [22]. After IR or interaction with Egf, Egfr translocalizes into the nucleus and interacts with and phosphorylates histone H4 at Y72 (H4-Y72) to recruit histone methyltransferase to methylate H4-K20, thereby promoting DNA synthesis and repair [23]. In addition to promoting DNA repair by increasing the activity of DNA-PKcs [20], survivin also plays an essential role in proper amphitelic kinetochore-spindle assembly, and downregulation of survivin leads to polyploidization, DNA damage and cell cycle arrest [38]; whereas, the upregulation of survivin likely promotes escape from therapy-induced senescence directly since survivin is pivotal for cell division and is degraded by the anaphase-promoting complex/cyclosome essential for senescence [39]. miR-21 promotes repair of DNA DSB by increasing activities of DNA-PKcs and Cyclin D1 through targeting glycogen synthase kinase 3β (GSK3B) and CDC25A [37]. CHEK1 can induce the degradation of CDC25A protein to facilitate DNA DSB repair, hence the upregulation of both Chek1 and miR-21 by transient Hh activation in irradiated SMGs may synergize to promote DNA repair. Hh signaling regulates the expression of Egfr and miR-21 in various cancer cells, but it is unclear whether they are direct target genes of Hh pathway. In irradiated liver, miR-21 expression is stimulated by EGFR/STAT3 pathway [40], suggesting that the upregulation of Egfr by Hh activation in SMGs might contribute to the induction of miR-21.
GDF15 is a member of the transforming growth factor β superfamily and is expressed in almost all tissues. GDF15 expression is upregulated in various pathological conditions such as inflammation [41], mitochondrial disease [42], and IR [29, 30]. The biological functions of GDF15 in IR damages appear to be dependent on cellular context. In endothelial cells, GDF15 overexpression leads to cellular senescence by increasing oxidative stress, whereas GDF15 knockdown reversed IR-induced cellular senescence [31]. In head and neck cancer cells, however, GDF15 was reported to contribute to radioresistance and cancer stemness by decreasing ROS via a SMAD-associated signaling pathway [43, 44]. We confirmed that the functions of GDF15 in HSG human salivary gland epithelial cells are similar to that in endothelial cells. Moreover, GDF15 can increase DNA methylation of miR-21 gene [35] that likely inhibits miR-21 expression; hence, the repression of GDF15 by miR-21 might further promote miR-21 expression following transient activation of Hh pathway to form a positive feedback loop.
IR-induced cellular senescence is well established by 2 weeks after IR in mouse models as indicated by the peak of the SASP factor IL6 in SMGs and serum [12], and senescence is typically irreversible after the presence of SASP as the result of extensive chromatin remodeling [45]. We reported that the transient expression of Shh transgene shortly (3 days) after IR achieved significantly better preservation of salivary gland function compared to expression long (30 or 90 days) after IR in mouse models [10, 11], which is likely due to the effective inhibition of IR-induced cellular senescence by Shh expression at early but not later time points. However, other mechanisms including preservation of putative stem/progenitor cells, parasympathetic innervation and microvessels also contribute to rescue effects of transient Shh expression, and Shh gene expression at later time points still significantly improved saliva secretion. Since the expression of Shh transgene needs be transient to avoid potential pro-tumor effects of sustained activation of Hh signaling, it will be worth examining whether repeated transient Hh activation at both early and later time points after IR will improve the rescue effects in future studies.
Conclusion
Transient activation of Hh signaling repressed IR-induced cellular senescence in salivary glands by enhancing DNA repair and decreasing oxidative stress, which contributes to the rescue of saliva-secreting function.
Abbreviations
IR: irradiation; Hh: Hedgehog; Shh: Sonic Hedgehog; SGs: salivary glands; SMGs: submandibular glands; GDF15: growth differentiation factor 15; qRT-PCR: quantitative reverse transcription polymerase chain reaction; HNC: head and neck cancer; miR-21: microRNA 21; GFP: green fluorescent protein; SA-β-gal: senescence-associated β-galactosidase; SASP: senescence-associated secretory phenotype; DSB: double strand breaks; γH2AX: phosphorylated H2AX; NHEJ: non-homologous end joining; HRR: homologous recombination repair; ROS: reactive oxygen species; SOD: superoxide dismutase; MDA: malondialdehyde; IF: immunofluorescence; shRNA: short hairpin RNA; SCsh: scrambled shRNAs; NT: non-treated; BSA: bovine serum albumin; IL6: interleukin 6.
Acknowledgements
This study was supported by NIH/NIDCR 1R01DE022975-01 (F.L.). The study was conceived and supervised by F.L.; B.H. performed most of the experiments; Q.Z. contributed to preparation of viral vectors and qRT-PCR; M.A.D. performed irradiation of animals; F.L. wrote the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29
2. Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T. et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220-41
3. Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN. et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1061-79
4. Ghosh-Laskar S, Yathiraj PH, Dutta D, Rangarajan V, Purandare N, Gupta T. et al. Prospective randomized controlled trial to compare 3-dimensional conformal radiotherapy to intensity-modulated radiotherapy in head and neck squamous cell carcinoma: Long-term results. Head Neck. 2016;38(Suppl 1):E1481-7
5. Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62:1187-94
6. Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ. et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494
7. Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007;15:2101-6
8. Mizukoshi K, Koyama N, Hayashi T, Zheng L, Matsuura S, Kashimata M. Shh/Ptch and EGF/ErbB cooperatively regulate branching morphogenesis of fetal mouse submandibular glands. Dev Biol. 2016;412:278-87
9. Hai B, Yang Z, Millar SE, Choi YS, Taketo MM, Nagy A. et al. Wnt/beta-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev. 2010;19:1793-801
10. Hai B, Qin L, Yang Z, Zhao Q, Shangguan L, Ti X. et al. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin Cancer Res. 2014;20:140-50
11. Hai B, Zhao Q, Qin L, Rangaraj D, Gutti VR, Liu F. Rescue Effects and Underlying Mechanisms of Intragland Shh Gene Delivery on Irradiation-Induced Hyposalivation. Hum Gene Ther. 2016;27:390-9
12. Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, Cohen J. et al. Radiation-Induced Loss of Salivary Gland Function Is Driven by Cellular Senescence and Prevented by IL6 Modulation. Cancer Res. 2016;76:1170-80
13. Sato M, Hayashi Y, Yoshida H, Yanagawa T, Yura Y, Nitta T. Search for specific markers of neoplastic epithelial duct and myoepithelial cell lines established from human salivary gland and characterization of their growth in vitro. Cancer. 1984;54:2959-67
14. Subkhankulova T, Zhang X, Leung C, Marino S. Bmi1 directly represses p21Waf1/Cip1 in Shh-induced proliferation of cerebellar granule cell progenitors. Mol Cell Neurosci. 2010;45:151-62
15. Zheng X, Wang Y, Liu B, Liu C, Liu D, Zhu J. et al. Bmi-1-shRNA inhibits the proliferation of lung adenocarcinoma cells by blocking the G1/S phase through decreasing cyclin D1 and increasing p21/p27 levels. Nucleic acid therapeutics. 2014;24:210-6
16. Tripathi K, Mani C, Barnett R, Nalluri S, Bachaboina L, Rocconi RP. et al. Gli1 protein regulates the S-phase checkpoint in tumor cells via Bid protein, and its inhibition sensitizes to DNA topoisomerase 1 inhibitors. J Biol Chem. 2014;289:31513-25
17. Faiao-Flores F, Alves-Fernandes DK, Pennacchi PC, Sandri S, Vicente AL, Scapulatempo-Neto C. et al. Targeting the hedgehog transcription factors GLI1 and GLI2 restores sensitivity to vemurafenib-resistant human melanoma cells. Oncogene. 2017;36:1849-61
18. Mazumdar T, DeVecchio J, Agyeman A, Shi T, Houghton JA. The GLI genes as the molecular switch in disrupting Hedgehog signaling in colon cancer. Oncotarget. 2011;2:638-45
19. Unruhe B, Schroder E, Wunsch D, Knauer SK. An Old Flame Never Dies: Survivin in Cancer and Cellular Senescence. Gerontology. 2016;62:173-81
20. Reichert S, Rodel C, Mirsch J, Harter PN, Tomicic MT, Mittelbronn M. et al. Survivin inhibition and DNA double-strand break repair: a molecular mechanism to overcome radioresistance in glioblastoma. Radiother Oncol. 2011;101:51-8
21. Vlckova K, Ondrusova L, Vachtenheim J, Reda J, Dundr P, Zadinova M. et al. Survivin, a novel target of the Hedgehog/GLI signaling pathway in human tumor cells. Cell death & disease. 2016;7:e2048
22. Yan S, Sorrell M, Berman Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell Mol Life Sci. 2014;71:3951-67
23. Chou RH, Wang YN, Hsieh YH, Li LY, Xia W, Chang WC. et al. EGFR modulates DNA synthesis and repair through Tyr phosphorylation of histone H4. Dev Cell. 2014;30:224-37
24. de la Cal C, Lomniczi A, Mohn CE, De Laurentiis A, Casal M, Chiarenza A. et al. Decrease in salivary secretion by radiation mediated by nitric oxide and prostaglandins. Neuroimmunomodulation. 2006;13:19-27
25. von Zglinicki T. Oxidative stress shortens telomeres. Trends in biochemical sciences. 2002;27:339-44
26. Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314:1918-22
27. Passos JF, Von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free radical research. 2006;40:1277-83
28. Chen KY, Chiu CH, Wang LC. Anti-apoptotic effects of Sonic hedgehog signalling through oxidative stress reduction in astrocytes co-cultured with excretory-secretory products of larval Angiostrongylus cantonensis. Scientific reports. 2017;7:41574
29. Tucker JD, Joiner MC, Thomas RA, Grever WE, Bakhmutsky MV, Chinkhota CN. et al. Accurate gene expression-based biodosimetry using a minimal set of human gene transcripts. Int J Radiat Oncol Biol Phys. 2014;88:933-9
30. Sandor N, Schilling-Toth B, Kis E, Benedek A, Lumniczky K, Safrany G. et al. Growth Differentiation Factor-15 (GDF-15) is a potential marker of radiation response and radiation sensitivity. Mutation research Genetic toxicology and environmental mutagenesis. 2015;793:142-9
31. Park H, Kim CH, Jeong JH, Park M, Kim KS. GDF15 contributes to radiation-induced senescence through the ROS-mediated p16 pathway in human endothelial cells. Oncotarget. 2016;7:9634-44
32. Iwasaki H, Nakano K, Shinkai K, Kunisawa Y, Hirahashi M, Oda Y. et al. Hedgehog Gli3 activator signal augments tumorigenicity of colorectal cancer via upregulation of adherence-related genes. Cancer Sci. 2013;104:328-36
33. Yang H, Filipovic Z, Brown D, Breit SN, Vassilev LT. Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation. Mol Cancer Ther. 2003;2:1023-9
34. Sandor N, Schilling-Toth B, Kis E, Fodor L, Mucsanyi F, Safrany G. et al. TP53inp1 Gene Is Implicated in Early Radiation Response in Human Fibroblast Cells. International journal of molecular sciences. 2015;16:25450-65
35. Ek WE, Hedman AK, Enroth S, Morris AP, Lindgren CM, Mahajan A. et al. Genome-wide DNA methylation study identifies genes associated with the cardiovascular biomarker GDF-15. Hum Mol Genet. 2016;25:817-27
36. Fu J, Rodova M, Nanta R, Meeker D, Van Veldhuizen PJ, Srivastava RK. et al. NPV-LDE-225 (Erismodegib) inhibits epithelial mesenchymal transition and self-renewal of glioblastoma initiating cells by regulating miR-21, miR-128, and miR-200. Neuro-oncology. 2013;15:691-706
37. Hu B, Wang X, Hu S, Ying X, Wang P, Zhang X. et al. miR-21-mediated Radioresistance Occurs via Promoting Repair of DNA Double Strand Breaks. J Biol Chem. 2017;292:3531-40
38. Wiedemuth R, Klink B, Topfer K, Schrock E, Schackert G, Tatsuka M. et al. Survivin safeguards chromosome numbers and protects from aneuploidy independently from p53. Mol Cancer. 2014;13:107
39. Wang Q, Wu PC, Roberson RS, Luk BV, Ivanova I, Chu E. et al. Survivin and escaping in therapy-induced cellular senescence. Int J Cancer. 2011;128:1546-58
40. Zhu Y, Yu X, Fu H, Wang H, Wang P, Zheng X. et al. MicroRNA-21 is involved in ionizing radiation-promoted liver carcinogenesis. International journal of clinical and experimental medicine. 2010;3:211-22
41. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M. et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847-58
42. Fujita Y, Ito M, Kojima T, Yatsuga S, Koga Y, Tanaka M. GDF15 is a novel biomarker to evaluate efficacy of pyruvate therapy for mitochondrial diseases. Mitochondrion. 2015;20:34-42
43. Schiegnitz E, Kammerer PW, Rode K, Schorn T, Brieger J, Al-Nawas B. Growth differentiation factor 15 as a radiation-induced marker in oral carcinoma increasing radiation resistance. J Oral Pathol Med. 2016;45:63-9
44. Li YL, Chang JT, Lee LY, Fan KH, Lu YC, Li YC. et al. GDF15 contributes to radioresistance and cancer stemness of head and neck cancer by regulating cellular reactive oxygen species via a SMAD-associated signaling pathway. Oncotarget. 2017;8:1508-28
45. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966-72
Author contact
![]() Corresponding author: F.L. (fliutamhsc.edu). Address: 206 Olsen Blvd, 208A Reynolds Medical Bldg., College Station, TX 77843-1114.
Corresponding author: F.L. (fliutamhsc.edu). Address: 206 Olsen Blvd, 208A Reynolds Medical Bldg., College Station, TX 77843-1114.
 Global reach, higher impact
Global reach, higher impact