13.3
Impact Factor
Theranostics 2017; 7(19):4894-4908. doi:10.7150/thno.20820 This issue Cite
Research Paper
Cell-cycle-specific Cellular Responses to Sonoporation
1. Key Laboratory of Modern Acoustics (Nanjing University), Ministry of Education, Nanjing University, Nanjing 210093, China;
2. Collaborative Innovation Center of Advanced Microstructures, National Laboratory of Solid State Microstructure, Department of Physics, Nanjing University, Nanjing 210093, China.
3. Center for Industrial and Medical Ultrasound, University of Washington, Seattle, WA 98105, USA;
4. Department of Physics, University of Vermont, Burlington, VT05405, USA.
5. The State Key Laboratory of Acoustics, Chinese Academy of Science, Beijing 10080, China.
* These authors contributed equally to this work.
Abstract
Microbubble-mediated sonoporation has shown its great potential in facilitating intracellular uptake of gene/drugs and other therapeutic agents that are otherwise difficult to enter cells. However, the biophysical mechanisms underlying microbubble-cell interactions remain unclear. Particularly, it is still a major challenge to get a comprehensive understanding of the impact of cell cycle phase on the cellular responses simultaneously occurring in cell membrane and cytoskeleton induced by microbubble sonoporation.
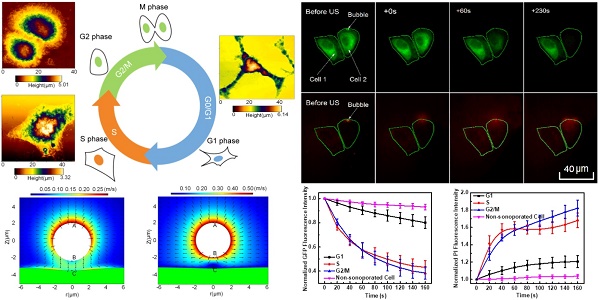
Methods: Here, efficient synchronizations were performed to arrest human cervical epithelial carcinoma (HeLa) cells in individual cycle phases. The, topography and stiffness of synchronized cells were examined using atomic force microscopy. The variations in cell membrane permeabilization and cytoskeleton arrangement induced by sonoporation were analyzed simultaneously by a real-time fluorescence imaging system.
Results: The results showed that G1-phase cells typically had the largest height and elastic modulus, while S-phase cells were generally the flattest and softest ones. Consequently, the S-Phase was found to be the preferred cycle for instantaneous sonoporation treatment, due to the greatest enhancement of membrane permeability and the fastest cytoskeleton disassembly at the early stage after sonoporation.
Conclusion: The current findings may benefit ongoing efforts aiming to pursue rational utilization of microbubble-mediated sonoporation in cell cycle-targeted gene/drug delivery for cancer therapy.
Keywords: Microbubble-mediated sonoporation, cell cycle, membrane permeabilization, cytoskeleton disassembly, bubble-cell-interaction
 Global reach, higher impact
Global reach, higher impact