13.3
Impact Factor
Theranostics 2017; 7(18):4424-4444. doi:10.7150/thno.18832 This issue Cite
Research Paper
Temperature-Sensitive Gold Nanoparticle-Coated Pluronic-PLL Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy
1. State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200032, People's Republic of China;
2. Department of General Surgery, School of Medicine, Xinhua Hospital, Shanghai Jiao Tong University, Shanghai 200092, People's Republic of China;
3. Department of Ophthalmology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, People's Republic of China.
* These authors contributed equally to this work.
Abstract
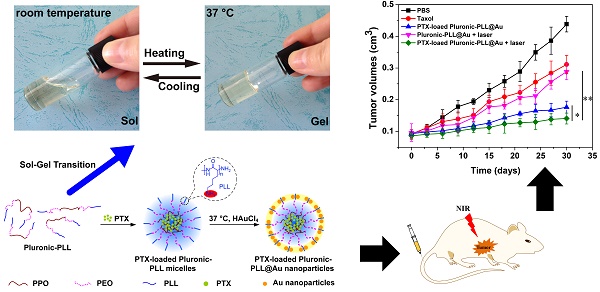
Gold nanoparticle-coated Pluronic-b-poly(L-lysine) nanoparticles (Pluronic-PLL@Au NPs) were synthesized via an easy one-step method and employed as carriers for the delivery of paclitaxel (PTX) in chemo-photothermal therapy, in which Pluronic-PLL acts as the reductant for the formation of AuNPs without the need for an additional reducing agent. Methods: The deposition of AuNPs on the surface of Pluronic-PLL micelles and the thermal response of the system were followed via ultraviolet-visible spectroscopy and dynamic light scattering. Calcein-AM and MTT assays were used to study the cell viability of MDA-MB-231 cells treated with PTX-loaded Pluronic-PLL@Au NPs, and we then irradiated the cells with NIR light. Results: An obvious temperature response was observed for the Pluronic-PLL@Au NPs. Blood compatibility and in vitro cytotoxicity assays confirmed that the Pluronic-PLL@Au NPs have excellent biocompatibility. Compared to Taxol, the PTX-loaded Pluronic-PLL@Au NPs exhibited higher cytotoxicity in MDA-MB-231 cells. All of these results and confocal laser scanning microscopy analysis results suggest that Pluronic-PLL@Au NPs greatly enhance the cellular uptake efficiency of the drug. Conclusion: As confirmed by in vitro and in vivo studies, the combination of chemotherapy and photothermal therapy can cause more damage than chemo- or photothermal therapy did alone, demonstrating the synergistic effect of chemo-photothermal treatment. Thus, the as-prepared Pluronic-PLL@Au NPs are promising for chemo-photothermal therapy.
Keywords: One-Pot Synthesis, Thermally Sensitive, Gold Nanoparticle, Pluronic-b-poly(L-lysine), Drug Delivery, Chemo-Photothermal.
 Global reach, higher impact
Global reach, higher impact