13.3
Impact Factor
Theranostics 2017; 7(7):1914-1927. doi:10.7150/thno.17852 This issue Cite
Research Paper
Triptolide Inhibits the AR Signaling Pathway to Suppress the Proliferation of Enzalutamide Resistant Prostate Cancer Cells
1. College of Life Sciences, Northwest A&F University, Yangling, Shaanxi, China
2. State Key Laboratory of Pharmaceutical Biotechnology and MOE Key Laboratory of Model Animals for Disease Study, Model Animal Research Center of Nanjing University, Nanjing, Jiangsu, China
3. Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
4. University of Chinese Academy of Sciences, Beijing 100049, China
5. Institute of Biophysics, Chinese Academy of Sciences, Chaoyang District, Beijing, China
# These authors contributed equally to this work.
Abstract
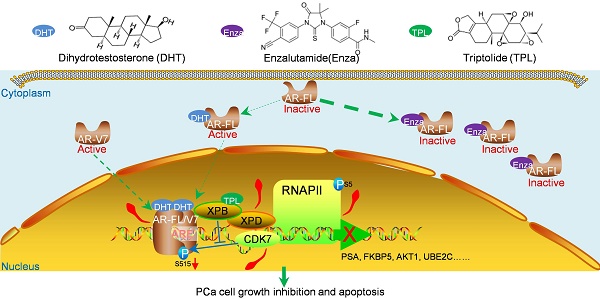
Enzalutamide is a second-generation androgen receptor (AR) antagonist for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Unfortunately, AR dysfunction means that resistance to enzalutamide will eventually develop. Thus, novel agents are urgently needed to treat this devastating disease. Triptolide (TPL), a key active compound extracted from the Chinese herb Thunder God Vine (Tripterygium wilfordii Hook F.), possesses anti-cancer activity in human prostate cancer cells. However, the effects of TPL against CRPC cells and the underlying mechanism of any such effect are unknown. In this study, we found that TPL at low dose inhibits the transactivation activity of both full-length and truncated AR without changing their protein levels. Interestingly, TPL inhibits phosphorylation of AR and its CRPC-associated variant AR-V7 at Ser515 through XPB/CDK7. As a result, TPL suppresses the binding of AR to promoter regions in AR target genes along with reduced TFIIH and RNA Pol II recruitment. Moreover, TPL at low dose reduces the viability of prostate cancer cells expressing AR or AR-Vs. Low-dose TPL also shows a synergistic effect with enzalutamide to inhibit CRPC cell survival in vitro, and enhances the anti-cancer effect of enzalutamide on CRPC xenografts with minimal side effects. Taken together, our data demonstrate that TPL targets the transactivation activity of both full-length and truncated ARs. Our results also suggest that TPL is a potential drug for CRPC, and can be used in combination with enzalutamide to treat CRPC.
Keywords: Triptolide, Enzalutamide, Castration-resistant prostate cancer, Androgen receptor, Phosphorylation.
 Global reach, higher impact
Global reach, higher impact