13.3
Impact Factor
Theranostics 2017; 7(7):1847-1862. doi:10.7150/thno.18930 This issue Cite
Review
Advances in the detection of telomerase activity using isothermal amplification
1. Faculty of Materials Science and Chemistry, China University of Geosciences, Wuhan 430074, P. R. China;
2. Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan 430074, P. R. China.
Received 2016-12-26; Accepted 2017-2-8; Published 2017-4-10
Abstract
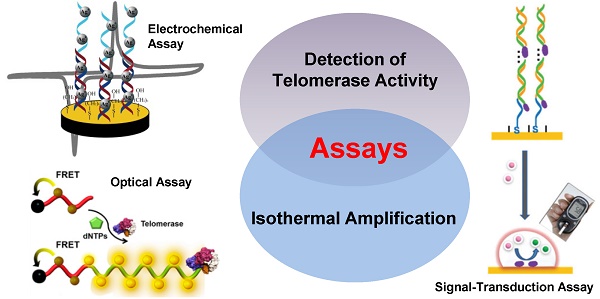
Telomerase plays a significantly important role in keeping the telomere length of a chromosome. Telomerase overexpresses in nearly all tumor cells, suggesting that telomerase could be not only a promising biomarker but also a potential therapeutic target for cancers. Therefore, numerous efforts focusing on the detection of telomerase activity have been reported from polymerase chain reaction (PCR)-based telomeric repeat amplification protocol (TRAP) assays to PCR-free assays such as isothermal amplification in recent decade. In this review, we highlight the strategies for the detection of telomerase activity using isothermal amplification and discuss some of the challenges in designing future telomerase assays as well.
Keywords: Telomerase activity, isothermal amplification, electrochemical assays, optical assays, signal-transduction assays.
Introduction
Telomerase, a ribonucleoprotein complex, is a specific reverse transcriptase and can elongate the telomeric repeats (TTAGGG) using the integral RNA as a template [1]. Its main role is to maintain the telomere length of a chromosome. To date, it is well-known that there is a great relationship between telomerase and cell death as well as carcinogenesis [2]. Telomerase plays an important role in the occurrence and development of cancers [3]. It was suggested that telomerase activity was in specific association with the oncogenic transformation of human cells [4]. The results indicated that telomerase activity could be detected in 85%-95% tumor cells of breast cancer, colon cancer, lymphoma, lung cancer, ovarian cancer, leukemia and so forth. Therefore, telomerase has become one of tumor biomarkers for judging the tumor cells [5]. The detection of telomerase activity is of particular importance in early diagnosis and treatment of cancers.
The detection of telomerase activity was early established by Morin et al. in 1989 using telomeric repeat elongation method [6]. The telomeric repeats were elongated through telomerase reverse transcription and telomeric template damage by adding RNase. The crude product was separated by polyacrylamide gel electrophoresis and imaged in positive bands by autoradiography. The method has good stability, but requires a large amount of samples, a complex experiment protocol, and a long testing time as well as the sensitivity is also poor. In 1994, Kim et al. established a telomeric repeat amplification protocol (TRAP) assay to elongate the telomeric repeats by polymerase chain reaction (PCR) [7]. The sensitivity was increased by about 104 times. Therefore, TRAP assays were widely used for the detection of telomerase activity in the past decade [8]. However, the traditional TRAP assays require the autoradiography of the amplified products by polyacrylamide gel electrophoresis and the use of radioactive label, which result in that their application in clinic is severely limited. Afterwards, a lot of the improved TRAP strategies were reported to simplify post-processing steps of time-consuming PCR [9]. For instance, molecular beacons [10], fluorescent dyes [11], and fluorescence resonance energy transfer (FRET) technology [12] were used to amplify PCR products.
PCR amplification requires thermal cycling instrumentation and the rebarbative cross contamination is difficult to be avoided. In order to overcome these shortcomings, isothermal amplification as an alternative of PCR because of its easy manipulation, low cost, PCR-like sensitivity, and quick results is established to detect tumor biomarkers including telomerase [13]. In the previous review, we have summarized the development of nanotechnologies for the detection of commercial biomarkers or extracted biomarkers using isothermal amplification [14]. The nanotechnologies are categorized into three parts according to the target-to-signal probe ratio in the detection strategy, namely, the N:N amplification ratio, the 1:N amplification ratio, and the 1:N2 amplification ratio. Here we summarize the assays for the detection of telomerase activity using isothermal amplification. Based on the detection technology, we divide the assays into three categories: electrochemical assays, optical assays, and signal-transduction assays. At last, we also discuss some of the challenges, for example, sensitivity and reliability, in designing future telomerase assays as well.
Assays for the Detection of Telomerase Activity Using Isothermal Amplification
Electrochemical Assays
Electrochemical assays are an attractive technique for the detection of telomerase activity because of their high sensitivity, low costs, and compatibility [15]. The innovative example is multiplexed electrical detection of tumor biomarkers with nanowire sensor arrays described by Lieber et al. in 2005 [16]. The elongation of the telomeric repeats on the silicon nanowire surface caused a change in conductance using silicon nanowires as a field transistor. To date, there is a great deal of electrochemical-based designs for the detection of telomerase activity. On the basis of the electrochemical signal source, we divide electrochemical assays into four categories: G-quadruplex/hemin DNAzyme-based electrochemical assays, nanoparticle-aided electrochemical assays, space-caused electrochemical assays, and electrochemiluminescence assays in the following discuss.
G-Quadruplex/Hemin DNAzyme-Based Electrochemical Assays
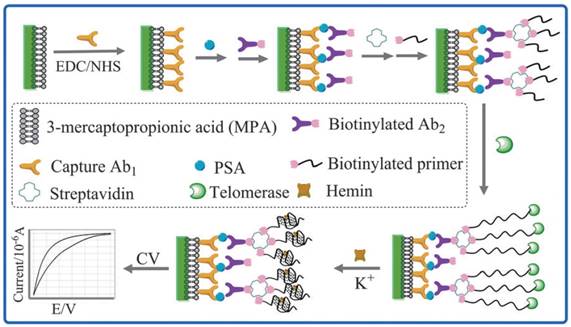
In 2004, Willner et al. firstly established a simple electrochemical method for the detection of telomerase activity using G-quadruplex/hemin DNAzyme without PCR amplification [17]. In the protocol, G-rich sequence was wrapped in the hairpin and template strand (TS) primer was located at the end of the hairpin. The elongated telomeric repeats from TS primer hybridized with the complementary strand DNA and the hybridization caused hairpin opening. The released G-rich sequence combined with hemin into G-quadruplex/hemin DNAzyme, which could catalyze the oxidation of 2,2'-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS) to generate green ABTS•+. Telomerase activity was calculated by measuring UV absorption of ABTS•+. The activity of G-quadruplex/hemin DNAzyme was then thoroughly investigated in 2011 [18]. The results indicated that the relationship between telomere length and DNAzyme activity is not direct. Afterwards, the detection methods based on G-quadruplex/hemin DNAzyme were gradually developed [19]. For example, Chen et al. developed a dual-functional electrochemical biosensor on a single electrode through combining G-quadruplex/hemin DNAzyme with the electrochemical immunosensors for the detection of prostate specific antigen (PSA) and telomerase activity together [20]. Both the sandwich immunoreaction and G-quadruplex/hemin DNAzyme were constructed on the gold electrode surface (Figure 1). First, primary antibody (Ab1) was coated on the gold electrode surface to capture PSA. PSA could catch biotinylated secondary antibody (Ab2) to form the sandwich immunoreaction. Steptavidin was then combined with the biotinylated immunocomplex to multiply bridge TS primer. The elongation of the telomeric repeats was initiated by telomerase to form a long single-strand DNA (ssDNA) using isothermal amplification. G-rich sequence in ssDNA and hemin formed G-quadruplex/hemin DNAzyme, which could catalyze the reduction of H2O2 to produce current measured by cyclic voltammetry (CV). The method could easily detect telomerase activity of prostate cancer and the PSA antigen in serum as well as the potential application in detecting other antigens. In addition, the high ability of DNA tetrahedron-structure was utilized to precisely regulate the assembly of G-quadruplex/hemin DNAzyme for polyaniline deposition on the gold electrode surface [21].
Schematic illustration of the dual-functional electrochemical assay based on G-quadruplex/hemin DNAzyme. Reprinted with permission from ref. [20]. Copyright (2013) Royal Society of Chemistry.
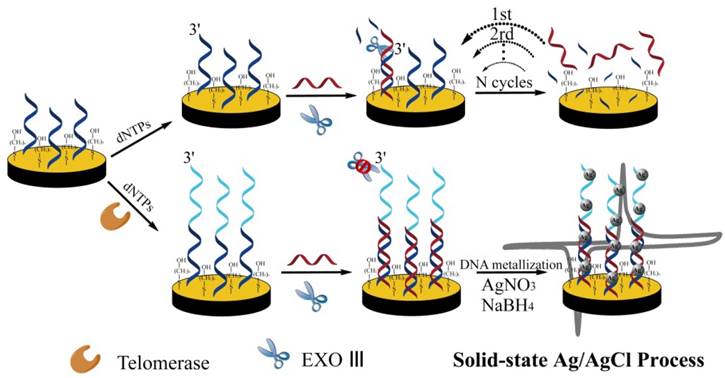
Schematic illustration of silver nanoparticles-aided electrochemical assay using highly specific solid-state electrochemical process. Reprinted with permission from ref. [23]. Copyright (2014) John Wiley & Sons.
Nanoparticle-Aided Electrochemical Assays
Electrocatalysis based on metal nanoparticle is a specific amplification strategy to enhance the signal in electrochemical biosensing [22]. For the detection of telomerase activity, Qu et al. developed DNA-silver nanoparticles (DNA-AgNPs) as an electroactive label using highly specific solid-state Ag/AgCl reaction [23]. TS primer was first immobilized on the gold electrode surface. The telomeric repeats were elongated in the presence of telomerase and dNTPs using isothermal amplification. Positively charged silver ions were bound to negatively charged DNA and then reduced by sodium borohydride to form AgNPs (Figure 2). This assay significantly decreased the detection limit through nanoparticle-mediated signal amplification. They also developed other nanoparticle-aided electrochemical assays based on superior catalytic property of platinum nanoparticles (PtNPs) [24, 25]. The electrochemical signal was derived from the hydrazine oxidation/hemoglobin reduction catalyzed by PtNPs. PtNPs were absorbed onto the glassy carbon electrode (GCE). Recently, PtNPs capsuled metal-organic frameworks (MOFs) onto a GCE provided a rapid electrochemical assay to detect telomerase activity in single HeLa cell [26].
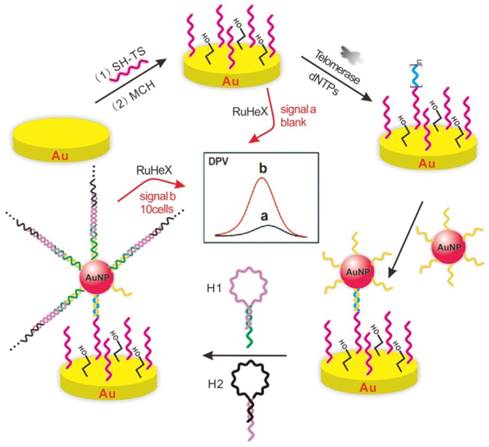
Nucleic acids-immobilized gold nanoparticles (AuNPs) are widely used for electrochemical signal amplification [27]. Zhu et al. captured spherical nucleic acids AuNPs through hybridization with the telomeric repeats on the gold electrode surface [28]. The hairpin probe alternated hybridization by the specific initiator on AuNPs to generate a nicked double helix for dual signal amplification. [Ru(NH3)6]3+ inserted into double helix via electrostatic interaction to further amplify the signal measured with differential pulse voltammetry (DPV) (Figure 3). Bio-barcode amplification assay using DNA-AuNPs conjugate as a kind of electrochemical method was applied to improve the sensitivity of the telomerase detection [29]. [Ru(NH3)6]3+ as an indicator complexed the phosphate ion of DNA by electrostatic interaction to generate electrochemical signal. This assay could detect the telomerase activity extracted from as little as 10 HeLa cells.
Liposome is able to load various biomarkers including enzymes and electroactive substances [30]. Dopamine-loaded biotinylated liposome was used to construct a highly sensitive electrochemical assay for the detection of telomerase activity in A549 cells [31]. In the study, carbon nanotubes-decorated GCE was used to enhance the sensitivity of the dopamine detection. Based on this strategy, the telomerase activity originated from 10 A549 cells could be detected.
Space-Caused Electrochemical Assays
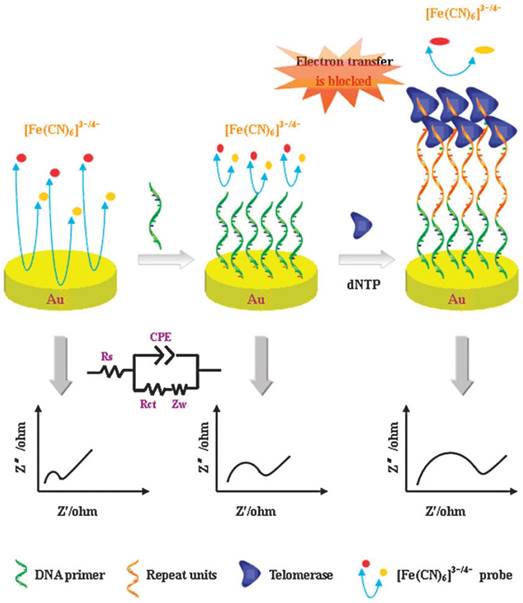
The space from the elongation of the telomeric repeats on the electrode will affect electron transfer process. Based on this phenomenon, Chen et al. reported a label-free detection method of telomerase activity by electrochemical impedance spectroscopy (EIS) using isothermal amplification [32]. TS primer immobilized on the gold electrode surface elongated the telomeric repeats to form a long ssDNA. The ssDNA blocked the electron transfer of Fe(CN)63-/Fe(CN)64- to the gold electrode surface (Figure 4). The intensity of the impedance is related to the telomerase activity. The method is simple, but the sensitivity remains to be raised to a higher level. Space-caused electrochemical probes were used to build a small biosensor microchip for real-time detection of telomerase activity in less than 20 minutes by electrochemical impedimetric biosensing [33]. Based on structure-switching DNA with ferrocene (Fc) as an electroactive indicator, space-caused electrochemical assay with a very wide linear response range, excellent stability and reproducibility was developed [34]. Moreover, the electrochemical oxidation signal intensity of guanine from the telomeric repeats on the gold electrode surface determined by DPV indicated the concentration of the telomerase [35]. The electrochemical assay in the limited space was recently proposed [36]. TS primer was first immobilized and repeating G-rich sequence was then formed using isothermal amplification on the inner wall of the nanochannels. Potassium ions (K+) induced the transformation of repeating G-rich sequence to multiplex G-quadruplex. Steric hindrance changes caused by transformation of repeating G-rich sequence in the porous anodic alumina nanochannels would affect the steady-state anodic current.
Schematic illustration of gold nanoparticles-aided dual signal amplification electrochemical assay. Reprinted with permission from ref. [28]. Copyright (2015) American Chemical Society.
Schematic illustration of space-caused electrochemical assay based on electrochemical impedance spectroscopy method. Reprinted with permission from ref. [32]. Copyright (2011) Royal Society of Chemistry.
Electrochemiluminescence Assays
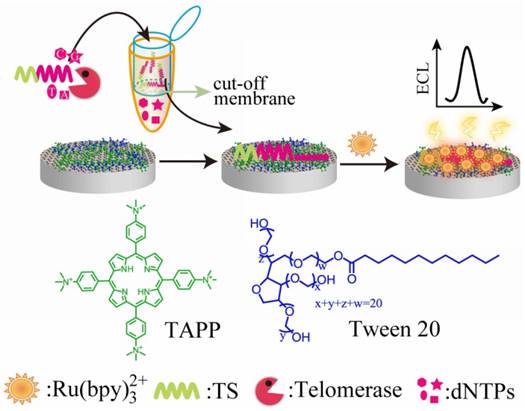
Electrochemiluminescence (ECL) is a highly sensitive technique for the detection of tumor biomarkers as well as telomerase [37]. ECL isothermal amplification assay for the detection of telomerase activity was early reported by Xing et al. [38]. ECL probe was hybridized to the telomerase elongation product, which was captured by streptavidin coated magnetic beads. ECL intensity is related to the concentration of telomerase. In addition, AuNPs on the electrode surface are able to enhance the luminescence signal and reduce the detection limit [39, 40]. Qu et al. constructed porphyrin-graphene nanocomposite modified GCE [41]. Meso-tetra(4-N,N,N-trimethylanilinium) porphyrin (TAPP), a cation porphyrin, inhibited graphene aggregation on the GCE surface. Tween 20 reduced nonspecific binding of proteins in physiological fluids. Ru(bpy)32+ was used as ECL signal reporter. The elongation of the telomeric repeats caused the dramatic increase in ECL signal of Ru(bpy)32+ because of the attraction between the telomeric repeats and Ru(bpy)32+ (Figure 5).
In addition to the above several assays, ferrocenylnaphthalene diimide as a tetraplex DNA-specific binder [42], horseradish peroxidase-conjugated avidin (avidin-HRP) for the electrochemical reduction of 3,3',5,5-tetramethylbenzidine (TMB) [43] and T7 exonuclease-aided 5'-methylene blue (MB) release [44] affected by telomerase elongation using isothermal amplification are also worthy of attention.
Optical Assays
Optical assays are the most widely employed strategy for the detection of telomerase activity [45-62]. Organic luminescent materials (molecular beacon, AIEgens) and inorganic nanomaterials (quantum dot, gold nanoparticle) which may release optical signal are used to monitor the telomerase product in vitro as well as in situ. Here we divide this part into four categories: fluorescent assays, chemiluminescent assays, colorimetric assays, and surface-enhanced Raman scattering (SERS) assays.
Schematic illustration of electrochemiluminenscence assay based on porphyrin-graphene nanocomposite-modified electrode. Reprinted with permission from ref. [41]. Copyright (2012) John Wiley & Sons.
Fluorescent Assays
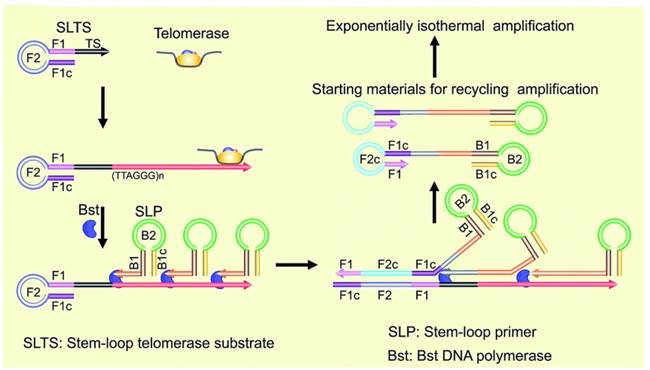
Unlike PCR-based amplification approach, isothermal amplification for fluorescent detection of telomerase activity can not only improve the sensitivity but also get rid of precise temperature control. A real-time assay by using exponential isothermal amplification of the telomeric repeats was developed [63]. In the presence of telomerase, TS primer may extend, initiating an exponential isothermal amplification reaction with the aid of two enzymes (nicking endonuclease, DNA polymerase) and two primers (TS primer and probe primer). As a result, ultrafast (25 min) and ultrasensitive (single HeLa cell) detection was achieved. Plaxco et al. reported a two-step and PCR-free assay for detecting telomerase activity by utilizing exonuclease III-aided target recycling. After isothermal amplification, target induced fluorescence emission change can be easily detected even by naked eye [64]. Recently, stem-loop primer-mediated exponential isothermal amplification strategy reported by Li et al. was used for accurately and specifically detecting telomerase activity [65]. By strategically designing two stem-loop structures, the assay can nonspecifically detect telomerase extracted from single cell. The stem-loop primer-mediated exponential amplification reaction can be achieved under isothermal circumstances with a single step and one enzyme (DNA polymerase) (Figure 6). Base on the elongation reaction (telomerase primer), strand-displacement property (polymerase) and inherent signal-transduction mechanism (hairpin fluorescence probe), telomerase activity was converted into fluorescence signal [66].
DNAzyme known as single strand of catalytic DNA sequence is a promising component for the analysis of targets in the biological and medical fields. Among them, 8-17 DNAzyme which could catalyze the cleavage of its complementary substrate with the aid of its corresponding metal ion cofactor is attracting growing interest as a high selectivity and sensitivity biosensor [67]. Tan et al. described a PbII-dependent 8-17 DNAzyme-based probe to detect the telomerase activity in cell lysates [68]. This single-molecule probe could detect total protein in cell lysate range from 0.1 to 20 μg and discriminate tumor cells from normal cells with a 5-fold signal enhancement. Furthermore, 8-17 DNAzyme-based in situ isothermal amplification assay was developed for detecting clinical sample or analyzing in situ histochemistry [69].
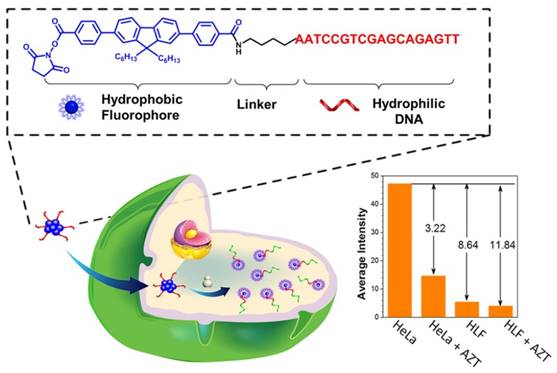
In order to avoid the interfering bio-background luminescence and biochemical degradation, Qu et al. constructed a cellulose paper-based platform with near infrared (NIR) excitable upconversion nanoparticle as a telomerase activity detection probe [70]. The solid-phase platform is stable enough to store the test results at room temperature thus can discriminate telomerase activity of different cell lines by the naked eye. Fluorescence polarization is a self-calibration method, which is less susceptible to many factors including fluorescence fluctuation, photobleaching and surrounding disturbance, making it a powerful technique for detection. More recently, Jin et al. present a fluorescence polarization strategy for isothermally amplified monitoring telomerase activity with high sensitivity and specificity [71]. In the presence of telomerase, the elongated telomeric repeats on the AuNPs hybridized with several short carboxyfluorescein (FAM)-modified complementary DNA to limit its molecular rotation, resulting in a high fluorescence polarization value. To simplify the structure and organic synthesis step, Xia et al. creatively designed a type of amphiphilic nucleic acid probes (ANAPs) comprised of a hydrophobic fluorene derivative unit and a hydrophilic DNA part, carrying on only fluorophore without any quencher [72]. In the absent of telomerase, the hydrophobic fluorophore unit in ANAPs aggregated, resulting in the fluorescent quenching according to aggregation-caused quenching effect (Figure 7). Upon binding with telomerase, the hydrophilic DNA part in ANAPs is elongated. Thus, the hydrophobic fluorophore unit disaggregates and liberates the fluorescent signal simultaneously. Owing to the high specificity of ANAPs, one-step isothermal amplification strategy is applied for tracking telomerase activity from not only mimic system but also clinic urine sample. Furthermore, ANAPs could distinguish tumor cells from normal cells by using efficient aggregation or dispersion [73].
Schematic illustration of stem-loop primer-mediated exponential isothermal amplification assay. Reprinted with permission from ref. [65]. Copyright (2016) Royal Society of Chemistry.
Schematic illustration of amphiphilic nucleic acid probes for the detection of telomerase activity. Reprinted with permission from ref. [72]. Copyright (2015) American Chemical Society.
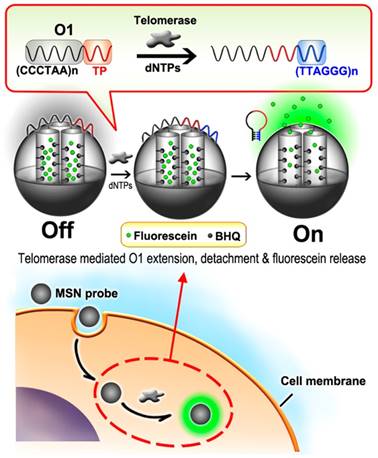
Nanomaterials such as mesoporous silica nanoparticle (MSN) bring us fantastic tools for real-time tracking of telomerase activity in living cells. For example, Ju et al. constructed a fluorescein contained MSN probe for intracellular mapping of telomerase activity [74]. Without telomerase, MSN probe was sealed by the wrapping DNA (O1, consists of TS primer and the telomeric repeats), fluorescence of fluorescein molecule was quenched by black hole fluorescence quencher (BHQ, modified on the inner wall of mesopore) (Figure 8). Active telomerase could trigger the extension and detachment of O1 from the MSN surface, leading to the gradual release of fluorescence observed from confocal microscopy image. This switchable and in situ strategy for tracking intracellular telomerase activity could distinguish different cell lines with various levels of telomerase activity and monitor the variation of its activity in response to telomerase inhibitor. In the following studies, they further simplified the procedure to make use of the nicked molecular beacon and gold nanoparticle for directly lighting up telomerase activity in living cells [75, 76].
Schematic illustration of mesoporous silica nanoparticle probe-based fluorescent assay. Reprinted with permission from ref. [74]. Copyright (2013) American Chemical Society.
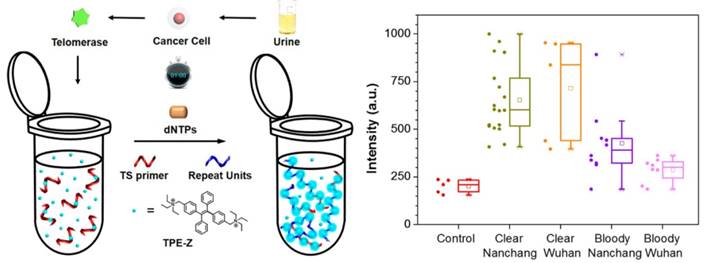
Recently, a photophysical phenomenon named aggregation-induced emission (AIE) was found in a group of fluorogens. Instead of aggregation-caused quenching (ACQ), the fluorescence intensity of fluorogens with AIE property is increased when AIE molecules are supramolecularly aggregated [77]. Xia and Lou et al. described an AIE-based turn-on technique for sensitive detection of telomerase activity [78]. A positively charged AIE dye, named as TPE-Z, is able to combine telomerase substrate oligonucleotides due to electrostatic attraction, but still shows weak emission owing to the finite physical constraint of short DNA strands (Figure 9). In the presence of active telomerase, the fluorescence intensity of TPE-Z could be remarkably increased after the elongation reaction and reflected the level of telomerase activity. This simple one-pot isothermal amplification strategy has been proved for the detection of telomerase activity in both clear (detection rate: 100%) or bloody (detection rate: 72%) urine samples from 41 bladder cancer patients and those from normal people. Although some improved achievements have been obtained, the specificity of the above assay is not satisfactory. To further enhance specificity, a quencher group was chemically modified on the primer to reduce the background noise and amplify the fluorescence signal [79]. As a result, this low background assay sustained excellent detection rate (100%) in clear urine specimens and achieved more satisfactory detection rate (87%) in bloody urine specimens. In order to improve the reproducibility, the combination of a cationic AIEgens with Cy3-labeled TS primer/FAM-labeled molecular beacon was performed for the detection of telomerase activity [80, 81].
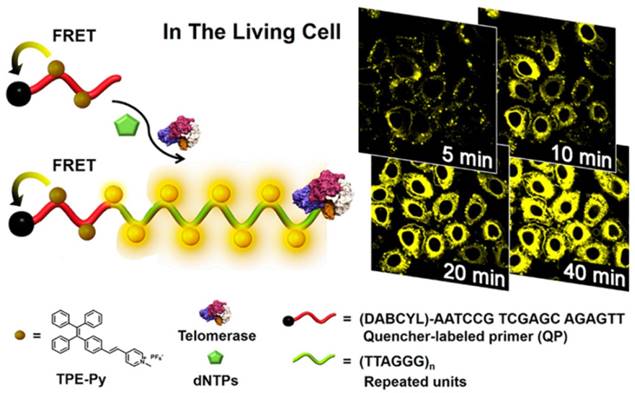
Besides the above AIEgens-based in vitro telomerase activity assays, imaging of telomerase activity with a turn-on manner in living cells has been also reported by Lou et al. [82]. They made use of a yellow-emissive AIE dye (TPE-Py) for in situ monitoring intracellular telomerase activity (Figure 10). Thanks to the electrostatic interaction, the positively charged TPE-Py could bind to the negatively charged telomerase substrate modified with a quencher group. Before telomerase related extension, TPE-Py emit faintly due to FRET process from the TPE-Py aggregation to the quencher. In living cells, telomerase substrate could be extended in the presence of active telomerase thus attract TPE-Py to fluoresce intensely. The proposed isothermal amplification assay exhibits remarkable advantages of broad applicability, superior photostability and feasibility for monitoring the variation of intracellular telomerase activity in different cell lines and screening telomerase-related drug as well. Recently, two positively charged AIEgens (Silole-R and TPE-H) as two fluorescent signals were united for enhanced monitor of extracellular and intracellular telomerase activity [83].
Schematic illustration of AIE-based one-pot fluorescent assay. Reprinted with permission from ref. [78]. Copyright (2015) American Chemical Society.
Schematic illustration of AIE-Based in situ fluorescent assay. Reprinted with permission from ref. [82]. Copyright (2016) American Chemical Society.
Chemiluminescent Assays
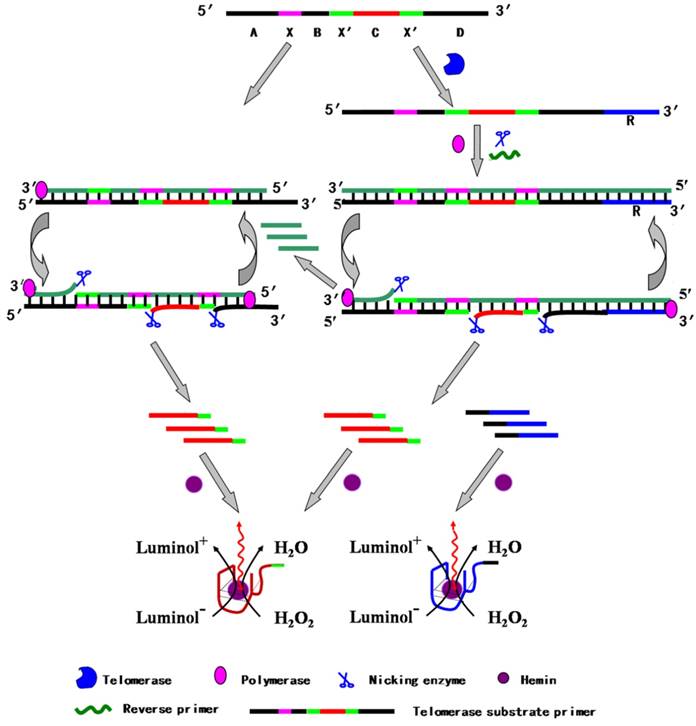
Luminol can be catalytically oxidized by H2O2 in the presence of G-quadruplex/hemin DNAzyme to generate chemiluminescence signal. With the advantages of simple procedure, the reduced nonspecific adsorption and high sensitivity, Zhang et al. developed a chemiluminescent assay for the detection of telomerase activity [84]. In the telomerization reaction step, substrate primer extended and produced several repeat units. The elongated product hybridized with the reverse primer, initiating strand displacement amplification (SDA) to generate short oligonucleotides (Figure 11). Afterwards, this short oligonucleotide might combine TS primer and consequently trigger an isothermally exponential amplification reaction (EXPAR), generating numerous catalytic DNAzyme sequence. Hemin could interact with DNAzyme sequence to form G-quadruplex structure, which could catalyze luminol with the aid of H2O2 to form chemiluminescent signal. On the contrary, both SDA and EXPAR are forbidden in the absence of active telomerase, failing to generate telomerase-related signal. Combination of wide dynamic range, highly sensitive chemiluminescent assay and highly efficient two-stage isothermal amplification, this method could sensitively detect telomerase activity from single HeLa cell with label-free DNA probe.
Colorimetric Assays
AuNPs have benefited the development of colorimetric assays for telomerase activity detection. For instance, Willner et al. reported a colorimetric sensing platform based on L-cysteine induced AuNPs aggregation [85]. In the presence of hemin and K+, guanosine-rich telomerase elongation repeat units could fold into G-quadruplex structure (Figure 12). This structure as horseradish peroxidase-like DNAzyme could catalyze the oxidation of L-cysteine (thiols as reactant) into cysteine (disulfides as product). At this moment, the UV absorption of AuNPs is prohibited to change from individual induced red into aggregated induced blue, which is highly correlated with the concentration (activity) of telomerase. Although the relatively fast detection time (3 h) and acceptable detection limit (27 cells/μL), the assay provides a potential point-of-care testing platform for cancer diagnosis observed by UV-vis spectrometry even naked eye.
Interestingly, Xia et al. developed a direct and bidirectional method for the detection of urine telomerase by making use of difunctional AuNPs with multiple colorimetric signals [86]. Difunctional AuNPs contain two sequences consist of telomerase primer and a reporter to combine the telomerase elongated repeats (Figure 13). Adding of active telomerase, the elongated repeats in the first kind of AuNPs could hybridize with the reporter from the second kind of AuNPs, forming a complex network structure. According to the concentration (activity) of telomerase, difunctional AuNPs exhibit four detection-color states including blue, red, purple and precipitate. The clinical diagnosis applicability of this accurate and simply assay has been proved by 18 urine specimens from inflammation patients, bladder cancer patients as well as healthy individuals. One of the advantages of the difunctional AuNPs-based assay is that the sensing events can be recognized by naked eye without complicated manipulation and sophisticated readout instrumentation.
Schematic illustration of telomere-induced two-stage isothermal amplification-mediated chemiluminescent assay. Reprinted with permission from ref. [84]. Copyright (2013) American Chemical Society.
Schematic illustration of a colorimetric assay using L-cysteine-mediated AuNPs aggregation. Reprinted with permission from ref. [85]. Copyright (2014) American Chemical Society.
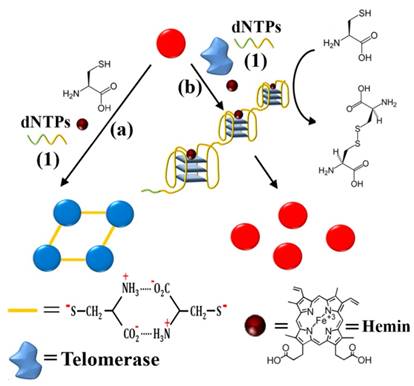
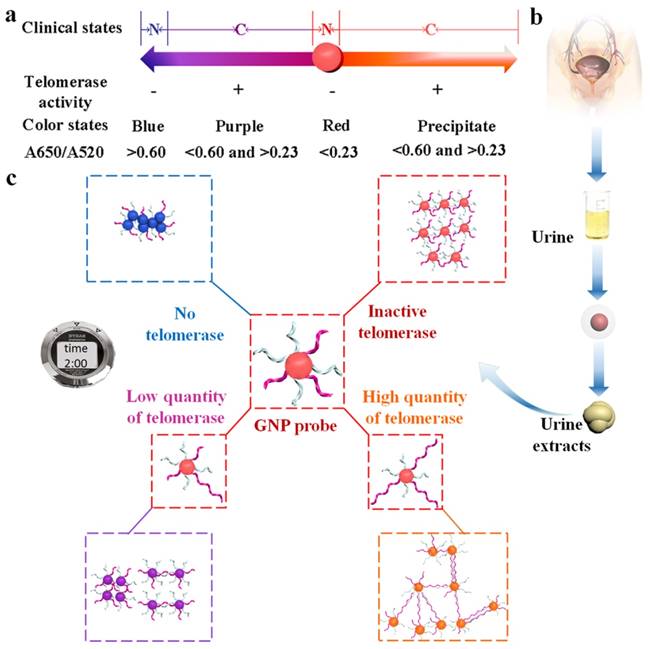
Schematic illustration of a colorimetric assay using difunctional AuNPs. (a) The number axis theory for clinical estimate of bladder cancer without pain based on proposed method (N, normal individuals; C, bladder cancer). (b) Telomerase extracted from urine samples. (c) The principle of telomerase detection. Reprinted with permission from ref. [86]. Copyright (2014) American Chemical Society.
Surface-Enhanced Raman Scattering (SERS) Assays
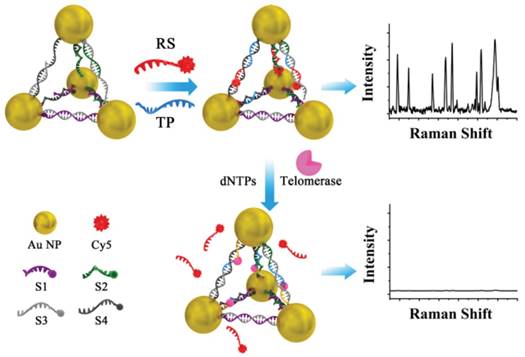
SERS has a good specificity for the identification of biological molecules and can detect the low concentration analysts. In recent years, SERS has got great development in biomedical imaging and disease diagnosis as well as the detection of telomerase activity due to the development of probe molecules and nanomaterials [87]. For example, 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) anchored on AuNPs as SERS reporter [88, 89], the elongation of the telomeric repeats caused a definite difference in SERS signal. The cyanine 5 (Cy5)-modified DNA sequence embedded with AuNPs-DNA pyramids as SERS reporter [90], the elongation of the telomeric repeats triggered the release of Cy5-modified DNA sequence from the pyramids, which caused a significant decrease in SERS signal (Figure 14). Recently, Yang et al. developed a quadratic signal isothermal amplification approach for SERS detection of telomerase activity at single-cell level [91]. The elongated telomeric repeats on the silica microbeads (SiMBs) surface hybridized the terminal sulfhydryl ssDNA to form a long double-strand DNA (dsDNA). The dsDNA subsequently captured AgNPs via Ag-S bond. Ag+ from the dissolution of AgNPs caused the aggregation of 4-aminobenzenthiol (4-ABT) immobilized on the AuNPs surface (AuNPs@4-ABT) to generate a lot of “hot-spots” for quadratic signal isothermal amplification using SERS signal as the readout. The strategy could achieve the detection limit down to single HeLa cell. Of note, this method probably has good performance at colon cancer tissues.
Schematic illustration of an SERS assay 5 using Cy5-modified DNA sequence embedded with AuNPs-DNA pyramids as SERS reporter. Reprinted with permission from ref. [90]. Copyright (2016) John Wiley & Sons.
Signal-Transduction Assays
Personal glucose meter has been effectively employed for the quantitative detection of glucose as well as biomarkers in the human blood [92]. As a signal output, personal glucose meter could be also used to detect non-glucose compounds such as DNA [93], small molecule [94], and protein [95]. It is a remarkable fact that personal glucose meter has been considered as a promising signal output candidate for the detection of telomerase activity. For example, biotin-modified TS capture probe tailored in a 96-well plate was elongated by telomerase and the elongated sequence hybridized the invertase-conjugated complementary probe to form a nick. The invertase turned sucrose to glucose, which generated signal readout by personal glucose meter [96]. The authors performed the signal-transduction detection of telomerase activity in 5 different cell lines (HeLa, A549, K562, MCF-7, and MDA-MB-231). The detection limit is under 20 HeLa cells. However, the authors also point out at last that this method has the false positive results for corresponding clinical use and more reliable result is probably got by the combination of other microscopic cytopathology methods in glucose meter readout for the detection of telomerase activity in tumor cells.
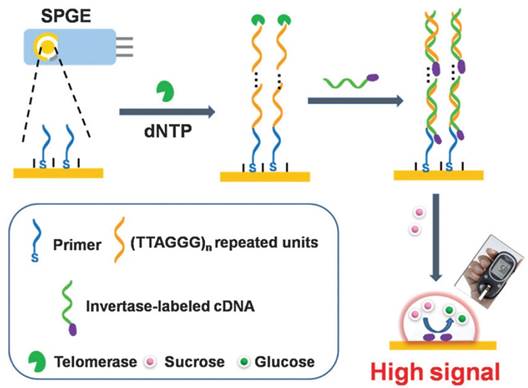
Yang et al. designed a tailored approach for the detection of telomerase activity using personal glucose meter as a signal transducer [97]. TS primer was first immobilized on commercially available screen-printed gold electrode surface via Au-S bond. Telomerase extracted from HeLa cells triggered the elongation reaction with the assistance of dNTPs using isothermal amplification to generate a long ssDNA. The hybridization of invertase-labeled cDNA introduced the invertase on the electrode. The conversation of sucrose to glucose by the invertase formed a strong readout signal from personal glucose meter (Figure 15). Furthermore, this portable detection method could be also used to investigate telomerase inhibitor.
Schematic illustration of signal-transduction assay using personal glucose meter as a signal transducer. Reprinted with permission from ref. [97]. Copyright (2014) Royal Society of Chemistry.
Conclusions and Outlook
In this review, we have summarized the recent development of several assays from tremendous efforts in focusing on the detection of telomerase activity using isothermal amplification. It makes sure that each method has its own advantages and disadvantages. For example, optical assays for the detection of telomerase activity are of relatively high throughput but relatively low sensitivity. Electrochemical assays have high sensitivity but require the use of electrochemical devices. Undoubtedly, the exploration of the assays for the detection of telomerase activity is still a highly important and extremely active research area in Analytical Chemistry. In the field of telomerase detection, several challenges (e.g. sensitivity and reliability) still remain if the assays are considered for application in fundamental laboratory research and clinical trials.
The sensitivity is one of the most important standards for evaluating analytical methods for detecting telomerase activity, which still puzzles the scientists because the telomerase molecule is at low concentration even in telomerase-positive tumor cells [98]. Therefore, tremendous efforts focusing on the sensitivity of the telomerase assays have been performed toward creating highly sensitive telomerase assays using isothermal amplification in the past decade [14]. Although some impressively sensitive methods have been reported, some key problems need to be concerned. For example, the primer extension assay may be enhanced to improve the sensitivity. The sensitivity for detecting telomerase activity in complex biological samples such as body fluid samples is still not good.
The reliability mainly is based upon the precise estimation of telomerase activity. Sometimes, an advanced telomerase assay is difficult to be repeated by other scientists because the technologies including external control experiment have not yet been standardized. In addition to methodology, the bioengineering process such as telomerase purification, assay handling, signal collection, and data analysis should be very important for quantitatively comparing control experiment and other reports. Precise estimation of telomerase activity will be also benefit for other unknown techniques.
In spite of sensitivity and reliability, the in vivo assays for the detection of telomerase activity still remain an enormous challenge. With the help of nanomaterials, the in vivo assays for the detection of telomerase activity may be achieved, but is still in the early stage [99]. Enormous efforts should be thrown in enhancing their performance including good selectivity and high sensitively in complex cellular environment. With the development of methodology, we believe that the in vivo assays for the detection of telomerase activity may achieve in clinical application for diagnosis of cancers in the foreseeable future.
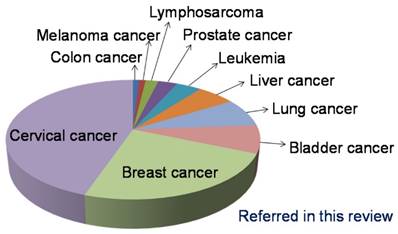
The assays for the detection of telomerase activity using isothermal amplification are the focal point in this review. They have been used to detect variety of cancers such as cervical cancer, breast cancer, bladder cancer, lung cancer, and so forth (Figure 16). But it should be strongly mentioned that telomerase could be not only a promising biomarker but also a potential therapeutic target for cancers. The anti-telomerase strategies such as using telomerase inhibitors are beneficial in treating tumors [100]. Various telomerase inhibitors have been well established for telomerase-based cancer therapy including pre-clinical studies. Therapeutic approaches mainly contain expression modulator, direct enzyme inhibitor, active immunotherapy, G-quadruplex inhibitor, and gene therapy. Telomerase inhibitors have the advantages of universal target, critical target, specificity, efficiency. However, there is a difference in the occurrence and development of different cancers, resulting in that there is not a universal inhibitor for the treatment of cancers. The use of telomerase inhibitors may induce non-telomerase elongation reaction. Telomerase inhibitors may have adverse side effects on human germ cells and hematopoietic stem cells in those a certain level of telomerase activity exists. It is still a great challenge that telomerase inhibitors are used in clinical for the treatment of cancers.
The occurrence frequency of cancer model studied in the assays for the detection of telomerase activity using isothermal amplification referred in this review.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21525523, 21574048, 21375042, 21405054), National Basic Research Program of China (973 program, 2015CB932600, 2013CB933000), and 1000 Young Talent (to Fan Xia).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569-73
2. Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10-8
3. Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21:349-53
4. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787-91
5. Hong B, Zu YL. Detecting circulating tumor cells: Current challenges and new trends. Theranostics. 2013;3:377-94
6. Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521-9
7. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PLC. et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-5
8. Zhou XM, Xing D. Assays for human telomerase activity: Progress and prospects. Chem Soc Rev. 2012;41:4643-56
9. Wang LJ, Ma F, Tang B, Zhang CY. Sensing telomerase: From in vitro detection to in vivo imaging. Chem Sci. 2017 DOI: 10.1039/C6SC04801C
10. Kong DM, Jin YW, Yin YJ, Mi HF, Shen HX. Real-time PCR detection of telomerase activity using specific molecular beacon probes. Anal Bioanal Chem. 2007;388:699-709
11. Gabourdes M, Bourgine V, Mathis G, Bazin H, Alpha-Bazin W. A homogeneous time-resolved fluorescence detection of telomerase activity. Anal Biochem. 2004;333:105-13
12. Xiao Y, Dane KY, Uzawa T, Csordas A, Qian JR, Soh HT. et al. Detection of telomerase activity in high concentration of cell lysates using primer-modified gold nanoparticles. J Am Chem Soc. 2010;132:15299-307
13. Zhao YX, Chen F, Li Q, Wang LH, Fan CH. Isothermal amplification of nucleic acids. Chem Rev. 2015;115:12491-545
14. Duan RX, Lou XD, Xia F. The development of nanostructure assisted isothermal amplification in biosensors. Chem Soc Rev. 2016;45:1738-49
15. Wu L, Qu XG. Cancer biomarker detection: Recent achievements and challenges. Chem Soc Rev. 2015;44:2963-97
16. Zheng GF, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005;23:1294-301
17. Xiao Y, Pavlov V, Niazov T, Dishon A, Kotler M, Willner I. Catalytic beacons for the detection of DNA and telomerase activity. J Am Chem Soc. 2004;126:7430-1
18. Stefan L, Denat F, Monchaud D. Deciphering the DNAzyme activity of multimeric quadruplexes: Insights into their actual role in the telomerase activity evaluation assay. J Am Chem Soc. 2011;133:20405-15
19. Onel B, Lin C, Yang DZ. DNA G-quadruplex and its potential as anticancer drug target. Sci China-Chem. 2014;57:1605-14
20. Liu J, Lu CY, Zhou H, Xu JJ, Wang ZH, Chen HY. A dual-functional electrochemical biosensor for the detection of prostate specific antigen and telomerase activity. Chem Commun. 2013;49:6602-4
21. Liu Y, Wei M, Liu X, Wei W, Zhao H, Zhang Y. et al. Label-free ultrasensitive detection of telomerase activity via multiple telomeric hemin/G-quadruplex triggered polyaniline deposition and a DNA tetrahedron-structure regulated signal. Chem Commun. 2016;52:1796-9
22. Pheeney CG, Guerra LF, Barton JK. DNA sensing by electrocatalysis with hemoglobin. Proc Natl Acad Sci U S A. 2012;109:11528-33
23. Wu L, Wang JS, Ren JS, Qu XG. Ultrasensitive telomerase activity detection in circulating tumor cells based on DNA metallization and sharp solid-state electrochemical techniques. Adv Funct Mater. 2014;24:2727-33
24. Zhang ZJ, Wu L, Wang JS, Ren JS, Qu XG. A Pt-nanoparticle electrocatalytic assay used for PCR-free sensitive telomerase detection. Chem Commun. 2013;49:9986-8
25. Wu L, Wang J, Sun H, Ren J, Qu X. Graphene-mesoporous silica-dispersed palladium nanoparticles-based probe carrier platform for electrocatalytic sensing of telomerase activity at less than single-cell level. Adv Healthc Mater. 2014;3:588-95
26. Ling PH, Lei JP, Jia L, Ju HX. Platinum nanoparticles encapsulated metal-organic frameworks for the electrochemical detection of telomerase activity. Chem Commun. 2016;52:1226-9
27. Zhang J, Song SP, Zhang LY, Wang LH, Wu HP, Pan D. et al. Sequence-specific detection of femtomolar DNA via a chronocoulometric DNA sensor (CDS): Effects of nanoparticle-mediated amplification and nanoscale control of DNA assembly at electrodes. J Am Chem Soc. 2006;128:8575-80
28. Wang WJ, Li JJ, Rui K, Gai PP, Zhang JR, Zhu JJ. Sensitive electrochemical detection of telomerase activity using spherical nucleic acids gold nanoparticles triggered mimic-hybridization chain reaction enzyme-free dual signal amplification. Anal Chem. 2015;87:3019-26
29. Li Y, Liu BW, Li X, Wei QL. Highly sensitive electrochemical detection of human telomerase activity based on bio-barcode method. Biosens Bioelectron. 2010;25:2543-7
30. Chumbimuni-Torres KY, Wu J, Clawson C, Galik M, Walter A, Flechsig GU. et al. Amplified potentiometric transduction of DNA hybridization using ion-loaded liposomes. Analyst. 2010;135:1618-23
31. Alizadeh-Ghodsi M, Zavari-Nematabad A, Hamishehkar H, Akbarzadeh A, Mahmoudi-Badiki T, Zarghami F. et al. Design and development of PCR-free highly sensitive electrochemical assay for detection of telomerase activity using Nano-based (liposomal) signal amplification platform. Biosens Bioelectron. 2016;80:426-32
32. Yang WQ, Zhu X, Liu QD, Lin ZY, Qiu B, Chen GN. Label-free detection of telomerase activity in HeLa cells using electrochemical impedance spectroscopy. Chem Commun. 2011;47:3129-31
33. Cunci L, Vargas MM, Cunci R, Gomez-Moreno R, Perez I, Baerga-Ortiz A. et al. Real-time detection of telomerase activity in cancer cells using a label-free electrochemical impedimetric biosensing microchip. RSC Adv. 2014;4:52357-65
34. Yi Z, Wang HB, Chen K, Gao Q, Tang H, Yu RQ. et al. A novel electrochemical biosensor for sensitive detection of telomerase activity based on structure-switching DNA. Biosens Bioelectron. 2014;53:310-5
35. Shao ZY, Liu YX, Xiao H, Li GX. PCR-free electrochemical assay of telomerase activity. Electrochem Commun. 2008;10:1502-4
36. Liu X, Wei M, Liu YJ, Lv BJ, Wei W, Zhang YJ. et al. Label-free detection of telomerase activity in urine using telomerase-responsive porous anodic alumina nanochannels. Anal Chem. 2016;88:8107-14
37. Miao WJ. Electrogenerated chemiluminescence and its biorelated applications. Chem Rev. 2008;108:2506-53
38. Zhou XM, Xing D, Zhu DB, Jia L. Magnetic bead and nanoparticle based electrochemiluminescence amplification assay for direct and sensitive measuring of telomerase activity. Anal Chem. 2009;81:255-61
39. Zhang HR, Wu MS, Xu JJ, Chen HY. Signal-on dual-potential electrochemiluminescence based on luminol-gold bifunctional nanoparticles for telomerase detection. Anal Chem. 2014;86:3834-40
40. Zhang HR, Wang YZ, Wu MS, Feng QM, Shi HW, Chen HY. et al. Visual electrochemiluminescence detection of telomerase activity based on multifunctional Au nanoparticles modified with G-quadruplex deoxyribozyme and luminol. Chem Commun. 2014;50:12575-7
41. Wu L, Wang JS, Feng LY, Ren JS, Wei WL, Qu XG. Label-free ultrasensitive detection of human telomerase activity using porphyrin-functionalized graphene and electrochemiluminescence technique. Adv Mater. 2012;24:2447-52
42. Sato S, Kondo H, Nojima T, Takenaka S. Electrochemical telomerase assay with ferrocenyl naphthalene diimide as a tetraplex DNA-specific binder. Anal Chem. 2005;77:7304-9
43. Li Y, Wen Y, Wang L, Liang W, Xu L, Ren S. et al. Analysis of telomerase activity based on a spired DNA tetrahedron TS primer. Biosens Bioelectron. 2015;67:364-9
44. Liu X, Li W, Hou T, Dong S, Yu G, Li F. Homogeneous electrochemical strategy for human telomerase activity assay at single-cell level based on T7 exonuclease-aided target recycling amplification. Anal Chem. 2015;87:4030-6
45. Ma Y, Wang ZH, Zhang M, Han ZH, Chen D, Zhu QY. et al. A telomerase-specific doxorubicin-releasing molecular beacon for cancer theranostics. Angew Chem-Int Edit. 2016;55:3304-8
46. Li XQ, Wang W, Chen YY, Ding CF. Fluorescence detection of telomerase activity in high concentration of cell lysates based on strand-displacement mediated recycling. Analyst. 2016;141:2388-91
47. Gao YF, Xu J, Li BX, Jin Y. PCR-free and label-free fluorescent detection of telomerase activity at single-cell level based on triple amplification. Biosens Bioelectron. 2016;81:415-22
48. Wang JS, Wu L, Ren JS, Qu XG. Visualizing human telomerase activity with primer-modified Au nanoparticles. Small. 2012;8:259-64
49. Wang JS, Wu L, Ren JS, Qu XG. Visual detection of telomerase activity with a tunable dynamic range by using a gold nanoparticle probe-based hybridization protection strategy. Nanoscale. 2014;6:1661-6
50. Zhang ZX, Sharon E, Freeman R, Liu XQ, Willner I. Fluorescence detection of DNA, adenosine-5 '-triphosphate (ATP), and telomerase activity by zinc(II)-protoporphyrin IX/G-quadruplex labels. Anal Chem. 2012;84:4789-97
51. Ding CF, Li XQ, Wang W, Chen YY. Fluorescence detection of telomerase activity in cancer cell extracts based on autonomous exonuclease III-assisted isothermal cycling signal amplification. Biosens Bioelectron. 2016;83:102-5
52. Sun PP, Ran X, Liu CQ, Liu CY, Pu F, Ren JS. et al. DNA-fueled molecular machine for label-free and non-enzymatic ultrasensitive detection of telomerase activity. Analyst. 2016;141:4855-8
53. Xu XL, Wei M, Liu YJ, Liu X, Wei W, Zhang YJ. et al. A simple, fast, label-free colorimetric method for detection of telomerase activity in urine by using hemin-graphene conjugates. Biosens Bioelectron. 2017;87:600-6
54. Peng L, Zhu Z, Chen Y, Han D, Tan WH. An exonuclease III and graphene oxide-aided assay for DNA detection. Biosens Bioelectron. 2012;35:475-8
55. Zhu GC, Yang K, Zhang CY. A single quantum dot-based biosensor for telomerase assay. Chem Commun. 2015;51:6808-11
56. Wang HB, Wu S, Chu X, Yu RQ. A sensitive fluorescence strategy for telomerase detection in cancer cells based on T7 exonuclease-assisted target recycling amplification. Chem Commun. 2012;48:5916-8
57. Zheng GF, Daniel WL, Mirkin CA. A new approach to amplified telomerase detection with polyvalent oligonucleotide nanoparticle conjugates. J Am Chem Soc. 2008;130:9644-5
58. Zhang XF, Cheng R, Shi ZL, Jin Y. A PCR-free fluorescence strategy for detecting telomerase activity via double amplification strategy. Biosens Bioelectron. 2016;75:101-7
59. Quach QH, Jung J, Kim H, Chung BH. A simple, fast and highly sensitive assay for the detection of telomerase activity. Chem Commun. 2013;49:6596-8
60. Hong M, Xu LD, Xue QW, Li L, Tang B. Fluorescence imaging of intracellular telomerase activity using enzyme-free signal amplification. Anal Chem. 2016;88:12177-82
61. Zhao YX, Qi L, Chen F, Zhao Y, Fan CH. Highly sensitive detection of telomerase activity in tumor cells by cascade isothermal signal amplification based on three-way junction and base-stacking hybridization. Biosens Bioelectron. 2013;41:764-70
62. Chen F, Zhang DX, Zhang Q, Zuo XL, Fan CH, Zhao YX. Zero-background helicase-dependent amplification and its application to reliable assay of telomerase activity in cancer cell by eliminating primer-dimer artifacts. ChemBioChem. 2016;17:1171-6
63. Tian LL, Weizmann Y. Real-time detection of telomerase activity using the exponential isothermal amplification of telomere repeat assay. J Am Chem Soc. 2013;135:1661-4
64. Zuo XL, Xia F, Patterson A, Soh HT, Xiao Y, Plaxco KW. Two-step, PCR-free telomerase detection by using exonuclease III-aided target recycling. ChemBioChem. 2011;12:2745-7
65. Wang HH, Wang H, Liu CH, Duan XR, Li ZP. Ultrasensitive detection of telomerase activity in a single cell using stem-loop primer-mediated exponential amplification (SPEA) with near zero nonspecific signal. Chem Sci. 2016;7:4945-50
66. Ding CF, Li XL, Ge Y, Zhang SS. Fluorescence detection of telomerase activity in cancer cells based on isothermal circular strand-displacement polymerization reaction. Anal Chem. 2010;82:2850-5
67. Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci U S A. 1997;94:4262-6
68. Wang H, Donovan MJ, Meng L, Zhao ZL, Kim YM, Ye M. et al. DNAzyme-based probes for telomerase detection in early-stage cancer diagnosis. Chem-Eur J. 2013;19:4633-9
69. Tian T, Peng S, Xiao H, Zhang XE, Guo S, Wang SR. et al. Highly sensitive detection of telomerase based on a DNAzyme strategy. Chem Commun. 2013;49:2652-4
70. Wang FM, Li W, Wang JS, Ren JS, Qu XG. Detection of telomerase on upconversion nanoparticle modified cellulose paper. Chem Commun. 2015;51:11630-3
71. Gao YF, Xu J, Li BX, Jin Y. Nanoparticle-aided amplification of fluorescence polarization for ultrasensitively monitoring activity of telomerase. ACS Appl Mater Interfaces. 2016;8:13707-13
72. Jia YM, Gao PC, Zhuang Y, Miao M, Lou XD, Xia F. Facile probe design: Fluorescent amphiphilic nucleic acid probes without quencher providing telomerase activity imaging inside living cells. Anal Chem. 2016;88:6621-6
73. Jia YM, Zuo XL, Lou XD, Miao M, Cheng Y, Min XH. et al. Rational designed bipolar, conjugated polymer-DNA composite beacon for the sensitive detection of proteins and ions. Anal Chem. 2015;87:3890-4
74. Qian RC, Ding L, Ju HX. Switchable fluorescent imaging of intracellular telomerase activity using telomerase-responsive mesoporous silica nanoparticle. J Am Chem Soc. 2013;135:13282-5
75. Qian RC, Ding L, Yan LW, Lin MF, Ju HX. Smart vesicle kit for in situ monitoring of intracellular telomerase activity using a telomerase-responsive probe. Anal Chem. 2014;86:8642-8
76. Qian RC, Ding L, Yan LW, Lin MF, Ju HX. A robust probe for lighting up intracellular telomerase via primer extension to open a nicked molecular beacon. J Am Chem Soc. 2014;136:8205-8
77. Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ. Aggregation-induced emission: Together we shine, united we soar!. Chem Rev. 2015;115:11718-940
78. Lou XD, Zhuang Y, Zuo XL, Jia YM, Hong YN, Min XH. et al. Real-time, quantitative lighting-up detection of telomerase in urines of bladder cancer patients by AIEgens. Anal Chem. 2015;87:6822-7
79. Zhuang Y, Zhang MS, Chen B, Duan RX, Min XH, Zhang ZY. et al. Quencher group induced high specificity detection of telomerase in clear and bloody urines by AlEgens. Anal Chem. 2015;87:9487-93
80. Zhuang Y, Xu Q, Huang FJ, Gao PC, Zhao ZJ, Lou XD. et al. Ratiometric fluorescent bioprobe for highly reproducible detection of telomerase in bloody urines of bladder cancer patients. ACS Sens. 2016;1:572-8
81. Ou XW, Hong F, Zhang ZY, Cheng Y, Zhao ZJ, Gao PC. et al. A highly sensitive and facile graphene oxide-based nucleic acid probe: Label-free detection of telomerase activity in cancer patient's urine using AIEgens. Biosens Bioelectron. 2017;89:417-21
82. Zhuang Y, Huang FJ, Xu Q, Zhang MS, Lou XD, Xia F. Facile, fast-responsive, and photostable imaging of telomerase activity in living cells with a fluorescence turn-on manner. Anal Chem. 2016;88:3289-94
83. Zhuang Y, Shang CL, Lou XD, Xia F. Construction of AIEgens-based bioprobe with two fluorescent signals for enhanced monitor of extracellular and intracellular telomerase activity. Anal Chem. 2017;89:2073-9
84. Wang LJ, Zhang Y, Zhang CY. Ultrasensitive detection of telomerase activity at the single-cell level. Anal Chem. 2013;85:11509-17
85. Sharon E, Golub E, Niazov-Elkan A, Balogh D, Willner I. Analysis of telomerase by the telomeric hemin/G-quadruplex-controlled aggregation of Au nanoparticles in the presence of cysteine. Anal Chem. 2014;86:3153-8
86. Duan RX, Wang BY, Zhang TC, Zhang ZY, Xu SF, Chen ZF. et al. Sensitive and bidirectional detection of urine telomerase based on the four detection-color states of difunctional gold nanoparticle probe. Anal Chem. 2014;86:9781-5
87. Granger JH, Schlotter NE, Crawford AC, Porter MD. Prospects for point-of-care pathogen diagnostics using surface-enhanced Raman scattering (SERS). Chem Soc Rev. 2016;45:3865-82
88. Zong SF, Wang ZY, Chen H, Cui YP. Ultrasensitive telomerase activity detection by telomeric elongation controlled surface enhanced Raman scattering. Small. 2013;9:4215-20
89. Zong SF, Wang ZY, Chen H, Hu GH, Liu M, Chen P. et al. Colorimetry and SERS dual-mode detection of telomerase activity: combining rapid screening with high sensitivity. Nanoscale. 2014;6:1808-16
90. Xu LG, Zhao S, Ma W, Wu XL, Li S, Kuang H. et al. Multigaps embedded nanoassemblies enhance in situ Raman spectroscopy for intracellular telomerase activity sensing. Adv Funct Mater. 2016;26:1602-8
91. Shi ML, Zheng J, Liu CH, Tan GX, Qing ZH, Yang S. et al. SERS assay of telomerase activity at single-cell level and colon cancer tissues via quadratic signal amplification. Biosens Bioelectron. 2016;77:673-80
92. Zhang JJ, Xiang Y, Wang M, Basu A, Lu Y. Dose-dependent response of personal glucose meters to nicotinamide coenzymes: Applications to point-of-care diagnostics of many non-glucose targets in a single step. Angew Chem-Int Edit. 2016;55:732-6
93. Xiang Y, Lu Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat Chem. 2011;3:697-703
94. Yang WX, Lu XH, Wang YC, Sun SJ, Liu CH, Li ZP. Portable and sensitive detection of protein kinase activity by using commercial personal glucose meter. Sens Actuator B-Chem. 2015;210:508-12
95. Xiang Y, Lu Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal Chem. 2012;84:4174-8
96. Wang WJ, Huang S, Li JJ, Rui K, Zhang JR, Zhu JJ. Coupling a DNA-based machine with glucometer readouts for amplified detection of telomerase activity in cancer cells. Sci Rep. 2016;6:23504
97. Zhu X, Xu HF, Lin RL, Yang GD, Lin ZY, Chen GN. Sensitive and portable detection of telomerase activity in HeLa cells using the personal glucose meter. Chem Commun. 2014;50:7897-9
98. Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850-3
99. Kim YH, Kim KT, Lee SJ, Hong SH, Moon JY, Yoon EK. et al. Image-aided suicide gene therapy utilizing multifunctional hTERT-targeting adenovirus for clinical translation in hepatocellular carcinoma. Theranostics. 2016;6:357-68
100. Romaniuk A, Kopczynski P, Ksiazek K, Rubis B. Telomerase modulation in therapeutic approach. Curr Pharm Design. 2014;20:6438-51
Author Biography
 Dr. Xiaojin Zhang is currently a professor at China University of Geosciences (Wuhan). He received his BSc degree (2007) and PhD degree (2012) from Wuhan University (Renxi Zhuo's group). He then worked as a postdoctoral fellow in Prof. Peter X. Ma's group at the University of Michigan, Ann Arbor. He joined China University of Geosciences (Wuhan) in 2016. His scientific interest is focused on nanomaterials for theranostics.
Dr. Xiaojin Zhang is currently a professor at China University of Geosciences (Wuhan). He received his BSc degree (2007) and PhD degree (2012) from Wuhan University (Renxi Zhuo's group). He then worked as a postdoctoral fellow in Prof. Peter X. Ma's group at the University of Michigan, Ann Arbor. He joined China University of Geosciences (Wuhan) in 2016. His scientific interest is focused on nanomaterials for theranostics.
 Dr. Xiaoding Lou is currently an associate professor at Huazhong University of Science and Technology (HUST). She received her PhD degree (2012) from Wuhan University (Zhen Li's group). She then worked as a Research Associate in Prof. Ben Zhong Tang's group at the Hong Kong University of Science and Technology. She joined HUST in 2013. Her scientific interest is focused on chemical and biosensor field.
Dr. Xiaoding Lou is currently an associate professor at Huazhong University of Science and Technology (HUST). She received her PhD degree (2012) from Wuhan University (Zhen Li's group). She then worked as a Research Associate in Prof. Ben Zhong Tang's group at the Hong Kong University of Science and Technology. She joined HUST in 2013. Her scientific interest is focused on chemical and biosensor field.
 Dr. Fan Xia is currently a professor at Huazhong University of Science and Technology (HUST) and Dean of Faculty of Materials Science and Chemistry, China University of Geosciences (Wuhan). He received his BSc degree (2003) from HUST and PhD degree (2008) from the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) (Lei Jiang's group). He then worked as a postdoctoral fellow in Prof. Alan J. Heeger's group at the University of California, Santa Barbara. He joined HUST as part of the 1000 Young Talents Program in 2012. His scientific interest is focused on bio-analytical chemistry.
Dr. Fan Xia is currently a professor at Huazhong University of Science and Technology (HUST) and Dean of Faculty of Materials Science and Chemistry, China University of Geosciences (Wuhan). He received his BSc degree (2003) from HUST and PhD degree (2008) from the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) (Lei Jiang's group). He then worked as a postdoctoral fellow in Prof. Alan J. Heeger's group at the University of California, Santa Barbara. He joined HUST as part of the 1000 Young Talents Program in 2012. His scientific interest is focused on bio-analytical chemistry.
![]() Corresponding authors: louxiaodingedu.cn (X.D. Lou); xiafanedu.cn (F. Xia).
Corresponding authors: louxiaodingedu.cn (X.D. Lou); xiafanedu.cn (F. Xia).
 Global reach, higher impact
Global reach, higher impact