13.3
Impact Factor
Theranostics 2017; 7(7):1806-1819. doi:10.7150/thno.18607 This issue Cite
Research Paper
Dual-pH Sensitive Charge-reversal Nanocomplex for Tumor-targeted Drug Delivery with Enhanced Anticancer Activity
1. Department of Pharmaceutical Analysis, School of Pharmacy, Fourth Military Medical University, Xi'an 710032, China;
2. Innovation experimental college, Northwest A&F University, Yangling 712100, China;
3. State Key Laboratory of Military Stomatology, Fourth Military Medical University, Xi'an 710032, China;
4. Department of Hepatobiliary Surgery, Xijing Hospital, Fourth Military Medical University, Xi'an 710032, China;
5. Department of Pharmaceutical Sciences, Irma Lerma Rangel College of Pharmacy, Texas A&M University Health Science Center, Kingsville, Texas 78363, United States.
* These authors contributed equally to this work.
Abstract
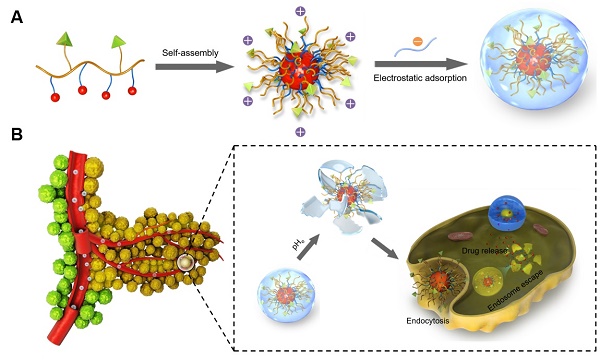
Poly(β-L-malic acid) (PMLA), a natural aliphatic polyester, has been proven to be a promising carrier for anti-cancer drugs. In spite of excellent bio-compatibility, the application of PMLA as the drug carrier for cancer therapy is limited by its low cellular uptake efficiency. The strong negative charge of PMLA impedes its uptake by cancer cells because of the electrostatic repulsion. In this study, a dual pH-sensitive charge-reversal PMLA-based nanocomplex (PMLA-PEI-DOX-TAT@PEG-DMMA) was developed for effective tumor-targeted drug delivery, enhanced cellular uptake, and intracellular drug release. The prepared nanocomplex showed a negative surface charge at the physiological pH, which could protect the nanocomplex from the attack of plasma proteins and recognition by the reticuloendothelial system, so as to prolong its circulation time. While at the tumor extracellular pH 6.8, the DMMA was hydrolyzed, leading to the charge reversal and exposure of the TAT on the polymeric micelles, thus enhancing the cellular internalization. Then, the polymeric micelles underwent dissociation and drug release in response to the acidic pH in the lyso/endosomal compartments of the tumor cell. Both in vitro and in vivo efficacy studies indicated that the nanocomplex significantly inhibited the tumor growth while the treatment showed negligible systemic toxicity, suggesting that the developed dual pH-sensitive PMLA-based nanocomplex would be a promising drug delivery system for tumor-targeted drug delivery with enhanced anticancer activity.
Keywords: PMLA, pH-sensitive, charge-reversal, TAT, cancer therapy.
 Global reach, higher impact
Global reach, higher impact