13.3
Impact Factor
Theranostics 2017; 7(3):624-633. doi:10.7150/thno.17484 This issue Cite
Research Paper
Hybrid Imaging Labels: Providing the Link Between Mass Spectrometry-Based Molecular Pathology and Theranostics
1. Interventional Molecular Imaging laboratory, Department of Radiology, Leiden University Medical Center, Leiden, the Netherlands;
2. Division of Molecular Pathology, Netherlands Cancer Institute- Antoni van Leeuwenhoek hospital (NKI-AvL), Amsterdam, the Netherlands.
3. Department of Analytical Chemistry, Ghent University, Ghent, Belgium.
4. Bundesanstalt für Materialforschung und -prüfung (BAM), Berlin, Germany.
5. Department of immunohematology and Blood Transfusion, Leiden University Medical Center, Leiden, the Netherlands.
6. RIKILT Wageningen UR, Wageningen, the Netherlands.
7. Center for Proteomics and Metabolomics, Leiden University Medical Center, Leiden, the Netherlands.
8. Laboratory of BioNanoTechnology, Wageningen University, Wageningen, the Netherlands.
Abstract
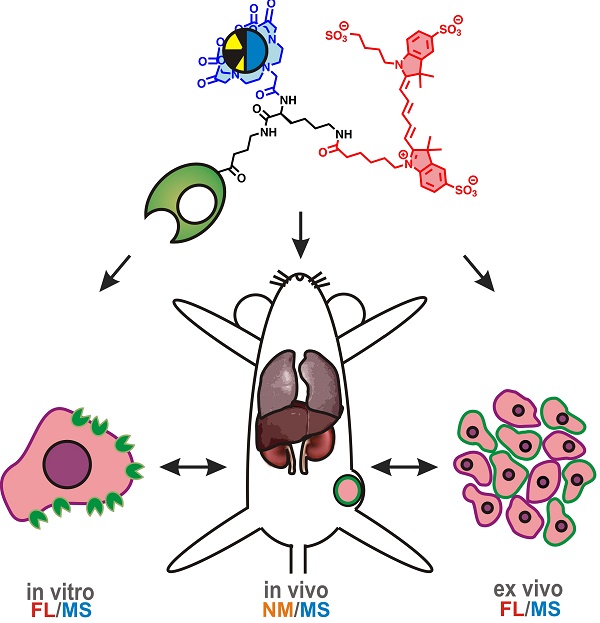
Background: Development of theranostic concepts that include inductively coupled plasma mass spectrometry (ICP-MS) and laser ablation ICP-MS (LA-ICP-MS) imaging can be hindered by the lack of a direct comparison to more standardly used methods for in vitro and in vivo evaluation; e.g. fluorescence or nuclear medicine. In this study a bimodal (or rather, hybrid) tracer that contains both a fluorescent dye and a chelate was used to evaluate the existence of a direct link between mass spectrometry (MS) and in vitro and in vivo molecular imaging findings using fluorescence and radioisotopes. At the same time, the hybrid label was used to determine whether the use of a single isotope label would allow for MS-based diagnostics.
Methods: A hybrid label that contained both a DTPA chelate (that was coordinated with either 165Ho or 111In) and a Cy5 fluorescent dye was coupled to the chemokine receptor 4 (CXCR4) targeting peptide Ac-TZ14011 (hybrid-Cy5-Ac-TZ4011). This receptor targeting tracer was used to 1) validate the efficacy of (165Ho-based) mass-cytometry in determining the receptor affinity via comparison with fluorescence-based flow cytometry (Cy5), 2) evaluate the microscopic binding pattern of the tracer in tumor cells using both fluorescence confocal imaging (Cy5) and LA-ICP-MS-imaging (165Ho), 3) compare in vivo biodistribution patterns obtained with ICP-MS (165Ho) and radiodetection (111In) after intravenous administration of hybrid-Cy5-Ac-TZ4011 in tumor-bearing mice. Finally, LA-ICP-MS-imaging (165Ho) was linked to fluorescence-based analysis of excised tissue samples (Cy5).
Results: Analysis with both mass-cytometry and flow cytometry revealed a similar receptor affinity, respectively 352 ± 141 nM and 245 ± 65 nM (p = 0.08), but with a much lower detection sensitivity for the first modality. In vitro LA-ICP-MS imaging (165Ho) enabled clear discrimination between CXCR4 positive and negative cells, but fluorescence microscopy was required to determine the intracellular distribution. In vivo biodistribution patterns obtained with ICP-MS (165Ho) and radiodetection (111In) of the hybrid peptide were shown to be similar. Assessment of tracer distribution in excised tissues revealed the location of tracer uptake with both LA-ICP-MS-imaging and fluorescence imaging.
Conclusion: Lanthanide-isotope chelation expands the scope of fluorescent/radioactive hybrid tracers to include MS-based analytical tools such as mass-cytometry, ICP-MS and LA-ICP-MS imaging in molecular pathology. In contradiction to common expectations, MS detection using a single chelate imaging agent was shown to be feasible, enabling a direct link between nuclear medicine-based imaging and theranostic methods.
Keywords: bimodal, fluorescence, molecular imaging, molecular pathology, mass spectrometry, mass cytometry, LA-ICP-MS imaging, radioisotopes, SPECT.
 Global reach, higher impact
Global reach, higher impact