13.3
Impact Factor
Theranostics 2019; 9(16):4779-4794. doi:10.7150/thno.32543 This issue Cite
Research Paper
The miR-561-5p/CX3CL1 Signaling Axis Regulates Pulmonary Metastasis in Hepatocellular Carcinoma Involving CX3CR1+ Natural Killer Cells Infiltration
1. Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai 200032, China; State Key Laboratory of Genetic Engineering, Fudan University, Shanghai, 200032, China
2. Key Laboratory of Carcinogenesis and Cancer Invasion, Fudan University, Ministry of Education, Shanghai, 200032, China
3. Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, 200032, China.
4. Department of Liver Surgery and Transplantation, Zhongshan Hospital, Fudan University, Shanghai, 200032, China
*These authors contribute to this work equally.
Abstract
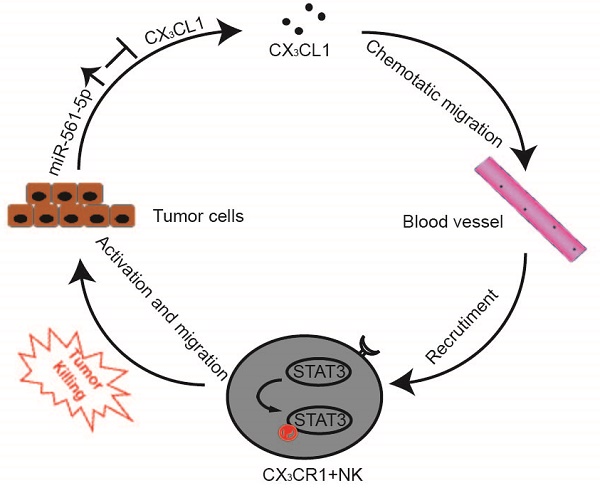
Natural killer (NK) cell can inhibit tumor initiation and regulates metastatic dissemination, acting as key mediators of the innate immune response. Intrinsic factors modulating NK cells infiltration and its anticancer activity remain poorly characterized. We investigated the roles of dysregulation of micro(mi)RNAs and NK cells in progression of hepatocellular carcinoma (HCC).
Methods: Small RNA sequencing were used to detect the miRNA profiles of tumor tissues from HCC patients with (n=14) or without (n=13) pulmonary metastasis and HCC cell lines with different pulmonary metastatic potentials. Chemokine expression profiling and bioinformatics were used to detect the downstream target of candidate target. In gain- and loss-of-function assays were used to investigate the role of miRNA in HCC progression. Different subsets of NK cells were isolated and used for chemotaxis and functional assays in vivo and in vitro. In situ hybridization and immunohistochemical analyses were performed to detect the expression of miRNA in tumor tissues from 242 HCC patients undergoing curative resection from 2010.
Results: Three miRNAs (miR-137, miR-149-5p, and miR-561-5p) were identified to be associated with pulmonary metastasis in patients with HCC. miR-561-5p was most highly overexpressed in metastatic HCC tissues and high-metastatic-potential HCC cell lines. In gain- and loss-of-function assays in a murine model, miR-561-5p promoted tumor growth and spread to the lungs. Yet, miR-561-5p did not appear to affect cellular proliferation and migration in vitro. Bioinformatics and chemokine expression profiling identified chemokine (C-X3-C motif) ligand 1 (CX3CL1) as a potential target of miR-561-5p. Furthermore, miR-561-5p promoted tumorigenesis and metastasis via CX3CL1-dependent regulation of CX3CR1+ NK cell infiltration and function. CX3CR1+ NK cells demonstrated stronger in vivo anti-metastatic activity relative to CX3CR1- NK cells. CX3CL1 stimulated chemotactic migration and cytotoxicity in CX3CR1+ NK cells via STAT3 signaling. Blockade of CX3CL1, CX3CR1, or of pSTAT3 signaling pathways attenuated the antitumor responses. Clinical samples exhibited a negative correlation between miR-561-5p expression and levels of CX3CL1 and CX3CR1+ NK cells. High miR-561-5p abundance, low CX3CL1 levels, and low numbers of CX3CR1+ NK cells were associated with adverse prognosis.
Conclusion: We delineated a miR-561-5p/CX3CL1/NK cell axis that drives HCC metastasis and demonstrated that CX3CR1+ NK cells serve as potent antitumor therapeutic effectors.
Keywords: HCC, tumor microenvironment, NK cell, chemokine, CX3CL1
 Global reach, higher impact
Global reach, higher impact