13.3
Impact Factor
Theranostics 2019; 9(6):1666-1682. doi:10.7150/thno.27891 This issue Cite
Research Paper
Membrane Radiolabelling of Exosomes for Comparative Biodistribution Analysis in Immunocompetent and Immunodeficient Mice - A Novel and Universal Approach
1. Institute of Pharmaceutical Science, Faculty of Life Sciences & Medicine, King's College London, Franklin-Wilkins Building, 150 Stamford Street, London SE1 9NH, United Kingdom
2. School of Medicine, Tenovus Building, University Hospital of Wales, Heath Park, Cardiff, CF14 4XN
3. School of Health Sport and Bioscience, University of East London, Water Lane, London E15 4LZ
4. Centre for Molecular Oncology, Barts Cancer Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ
Abstract
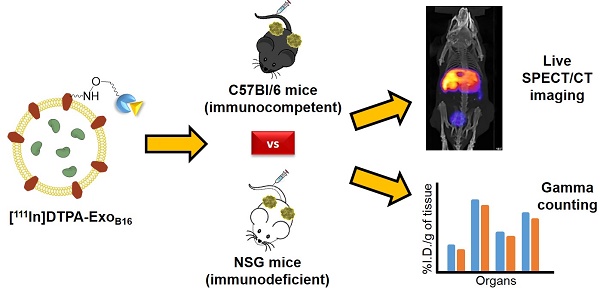
Extracellular vesicles, in particular exosomes, have recently gained interest as novel drug delivery vectors due to their biological origin and inherent intercellular biomolecule delivery capability. An in-depth knowledge of their in vivo biodistribution is therefore essential. This work aimed to develop a novel, reliable and universal method to radiolabel exosomes to study their in vivo biodistribution.
Methods: Melanoma (B16F10) cells were cultured in bioreactor flasks to increase exosome yield. B16F10-derived exosomes (ExoB16) were isolated using ultracentrifugation onto a single sucrose cushion, and were characterised for size, yield, purity, exosomal markers and morphology using nanoparticle tracking analysis (NTA), protein measurements, flow cytometry and electron microscopy. ExoB16 were radiolabelled using 2 different approaches - intraluminal labelling (entrapment of 111Indium via tropolone shuttling); and membrane labelling (chelation of 111Indium via covalently attached bifunctional chelator DTPA-anhydride). Labelling efficiency and stability was assessed using gel filtration and thin layer chromatography. Melanoma-bearing immunocompetent (C57BL/6) and immunodeficient (NSG) mice were injected intravenously with radiolabelled ExoB16 (1x1011 particles/mouse) followed by metabolic cages study, whole body SPECT-CT imaging and ex vivo gamma counting at 1, 4 and 24 h post-injection.
Results: Membrane-labelled ExoB16 showed superior radiolabelling efficiency and radiochemical stability (19.2 ± 4.53 % and 80.4 ± 1.6 % respectively) compared to the intraluminal-labelled exosomes (4.73 ± 0.39 % and 14.21 ± 2.76 % respectively). Using the membrane-labelling approach, the in vivo biodistribution of ExoB16 in melanoma-bearing C57Bl/6 mice was carried out, and was found to accumulate primarily in the liver and spleen (~56% and ~38% ID/gT respectively), followed by the kidneys (~3% ID/gT). ExoB16 showed minimal tumour i.e. self-tissue accumulation (~0.7% ID/gT). The membrane-labelling approach was also used to study ExoB16 biodistribution in melanoma-bearing immunocompromised (NSG) mice, to compare with that in the immunocompetent C57Bl/6 mice. Similar biodistribution profile was observed in both C57BL/6 and NSG mice, where prominent accumulation was seen in liver and spleen, apart from the significantly lower tumour accumulation observed in the NSG mice (~0.3% ID/gT).
Conclusion: Membrane radiolabelling of exosomes is a reliable approach that allows for accurate live imaging and quantitative biodistribution studies to be performed on potentially all exosome types without engineering parent cells.
Keywords: exosomes, drug delivery, radiolabelling, biodistribution
 Global reach, higher impact
Global reach, higher impact