13.3
Impact Factor
Theranostics 2019; 9(1):290-310. doi:10.7150/thno.28671 This issue Cite
Research Paper
Direct in vivo application of induced pluripotent stem cells is feasible and can be safe
1. Department of Physiology & Pathophysiology, School of Basic Medical Sciences, Fudan University, Shanghai, P.R. China
2. Department of Biomedical Engineering, The University of Alabama at Birmingham, Birmingham, USA
*These authors contributed equally.
Abstract
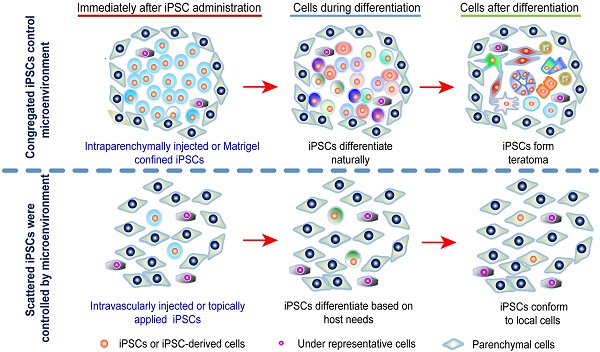
Increasing evidence suggests the consensus that direct in vivo application of induced pluripotent stem cells (iPSCs) is infeasible may not be true.
Methods: Teratoma formation and fate were examined in 53 normal and disease conditions involving brain, lung, liver, kidney, islet, skin, hind limb, and arteries.
Results: Using classic teratoma generation assays, which require iPSCs to be congregated and confined, all mouse, human, and individualized autologous monkey iPSCs tested formed teratoma, while iPSC-derived cells did not. Intravenously or topically-disseminated iPSCs did not form teratomas with doses up to 2.5×108 iPSCs/kg and observation times up to 18 months, regardless of host tissue type; autologous, syngeneic, or immune-deficient host animals; presence or absence of disease; disease type; iPSC induction method; commercial or self-induced iPSCs; mouse, human, or monkey iPSCs; frequency of delivery; and sex. Matrigel-confined, but not PBS-suspended, syngeneic iPSCs delivered into the peritoneal cavity or renal capsule formed teratomas. Intravenously administered iPSCs were therapeutic with a dose as low as 5×106/kg and some iPSCs differentiated into somatic cells in injured organs. Disseminated iPSCs trafficked into injured tissue and survived significantly longer in injured than uninjured organs. In disease-free animals, no intravenously administered cell differentiated into an unwanted long-lasting cell or survived as a quiescent stem cell. In coculture, the stem cell medium and dominant cell-type status were critical for iPSCs to form cell masses.
Conclusion: Teratoma can be easily and completely avoided by disseminating the cells. Direct in vivo iPSC application is feasible and can be safe.
Keywords: induced pluripotent stem cells, teratoma, cellular microenvironment, monkey
 Global reach, higher impact
Global reach, higher impact