13.3
Impact Factor
Theranostics 2018; 8(22):6367-6383. doi:10.7150/thno.28324 This issue Cite
Research Paper
Therapeutic Fluorescent Hybrid Nanoparticles for Traceable Delivery of Glucocorticoids to Inflammatory Sites
1. Institute of Diagnostic and Interventional Radiology, University Medicine Goettingen, Goettingen, Germany
2. Translational Molecular Imaging, Max-Planck Institute for Experimental Medicine, Goettingen, Germany
3. Institute of Inorganic Chemistry, Karlsruhe Institute of Technology, Karlsruhe, Germany
4. Italian Synchrotron “Elettra”, Trieste, Italy
5. Department of Neurogenetics, Max-Planck Institute for Experimental Medicine, Goettingen, Germany
6. Institute of Biological and Medical Imaging, Helmholtz Zentrum Munich, Germany and Chair of Biological Imaging, Technical University of Munich, Germany
7. Clinic of Haematology and Medical Oncology, University Medicine Goettingen, Goettingen, Germany
Abstract
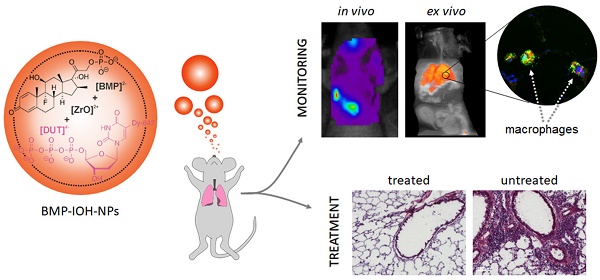
Treatment of inflammatory disorders with glucocorticoids (GCs) is often accompanied by severe adverse effects. Application of GCs via nanoparticles (NPs), especially those using simple formulations, could possibly improve their delivery to sites of inflammation and therefore their efficacy, minimising the required dose and thus reducing side effects. Here, we present the evaluation of NPs composed of GC betamethasone phosphate (BMP) and the fluorescent dye DY-647 (BMP-IOH-NPs) for improved treatment of inflammation with simultaneous in vivo monitoring of NP delivery.
Methods: BMP-IOH-NP uptake by MH-S macrophages was analysed by fluorescence and electron microscopy. Lipopolysaccharide (LPS)-stimulated cells were treated for 48 h with BMP-IOH-NPs (1×10-5-1×10-9 M), BMP or dexamethasone (Dexa). Drug efficacy was assessed by measurement of interleukin 6. Mice with Zymosan-A-induced paw inflammation were intraperitoneally treated with BMP-IOH-NPs (10 mg/kg) and mice with ovalbumin (OVA)-induced allergic airway inflammation (AAI) were treated intranasally with BMP-IOH-NPs, BMP or Dexa (each 2.5 mg/kg). Efficacy was assessed in vivo by paw volume measurements with µCT and ex vivo by measurement of paw weight for Zymosan-A-treated mice, or in the AAI model by in vivo x-ray-based lung function assessment and by cell counts in the bronchoalveolar lavage (BAL) fluid and histology. Delivery of BMP-IOH-NPs to the lungs of AAI mice was monitored by in vivo optical imaging and by fluorescence microscopy.
Results: Uptake of BMP-IOH-NPs by MH-S cells was observed during the first 10 min of incubation, with the NP load increasing over time. The anti-inflammatory effect of BMP-IOH-NPs in vitro was dose dependent and higher than that of Dexa or free BMP, confirming efficient release of the drug. In vivo, Zymosan-A-induced paw inflammation was significantly reduced in mice treated with BMP-IOH-NPs. AAI mice that received BMP-IOH-NPs or Dexa but not BMP revealed significantly decreased eosinophil numbers in BALs and reduced immune cell infiltration in lungs. Correspondingly, lung function parameters, which were strongly affected in non-treated AAI mice, were unaffected in AAI mice treated with BMP-IOH-NPs and resembled those of healthy animals. Accumulation of BMP-IOH-NPs within the lungs of AAI mice was detectable by optical imaging for at least 4 h in vivo, where they were preferentially taken up by peribronchial and alveolar M2 macrophages.
Conclusion: Our results show that BMP-IOH-NPs can effectively be applied in therapy of inflammatory diseases with at least equal efficacy as the gold standard Dexa, while their delivery can be simultaneously tracked in vivo by fluorescence imaging. BMP-IOH-NPs thus have the potential to reach clinical applications.
Keywords: hybrid nanoparticles, nanoparticle-based therapy, inflammatory disease, monitoring glucocorticoid delivery, in vivo imaging
 Global reach, higher impact
Global reach, higher impact