13.3
Impact Factor
Theranostics 2018; 8(22):6352-6354. doi:10.7150/thno.31515 This issue Cite
Editorial
Targeted nanoparticles for multimodal imaging of the receptor for advanced glycation end-products
Departments of Radiology and Medicinal Chemistry, University of Michigan, Ann Arbor, MI 48109, USA.
Received 2018-1-27; Accepted 2018-4-9; Published 2018-12-1
Commentary-article in Theranostics, Volume 8, 5012
Abstract
The receptor for advanced glycation end-products (RAGE) is implicated in multiple disease states such as cancer, diabetes and neurodegenerative disorders, and RAGE inhibitors are being explored as potential new therapies in such cases. Despite the known role RAGE plays in these conditions, there remains an urgent need for a molecular imaging agent that can accurately quantify RAGE levels in vivo, aid in validation of RAGE as a biomarker and/or therapeutic target, and support development of new RAGE inhibitors. This editorial highlights a multimodal nanoparticle-based imaging agent targeted at RAGE that was recently developed by Konopka and colleagues (Theranostics 2018; 8(18):5012-5024. doi:10.7150/thno.24791).
Keywords: receptor for advanced glycation end-products, multimodal imaging, positron emission tomography.
The receptor for advanced glycation end-products (RAGE) is a pattern-recognition receptor involved in several diseases and disorders because of its proinflammatory signaling mechanism. It recognizes a large variety of ligands including carboxymethyllysine (CML), a non-enzymatically glycated amino acid residue; RAGE-ligand binding promotes inflammation through the NF-κB pathway [2]. There are multiple splice-isoforms of RAGE which have been speculated to act opposite to the full length, membrane bound form of RAGE. The soluble esRAGE (formed from an alternative mRNA splicing) circulates and possibly acts as a scavenger receptor, sequestering ligands from the full length receptor [3]. The full length receptor is upregulated in the presence of its ligands, but little is understood about ectodomain shedding and the relationship with esRAGE in this state.
Reflecting its role in inflammation and implication in numerous disease states, RAGE has been investigated as a possible therapeutic target. For example, a recombinant form of soluble RAGE (sRAGE) was engineered to be used as a RAGE inhibitor [4], and inhibition of RAGE through this method was shown to reduce development and progression of many pathological states in animal models of diabetes [4], cardiovascular disease [5], and cancer [6]. However, therapeutic efforts to inhibit RAGE in clinical trials have had limited success to date; the small molecule Azeliragon (TTP-488) failed efficacy in phase II and phase III clinical trials in early Alzheimer's disease (AD) patients with mild cognitive impairment [7]. While antibody, peptide and DNA aptamer-based inhibitors have shown preclinical success, none have been translated into clinical use at this time.
Concurrent with efforts to develop therapeutic RAGE inhibitors, a number of groups have explored development of diagnostics targeting RAGE with the goal of using it as a biomarker. Diagnostic agents for RAGE would allow use of imaging to quantify the receptor in vivo, providing new information on the receptor's involvement in disease, and to support drug discovery efforts around RAGE. For example, a radiotracer for RAGE would allow confirmation of target engagement by new RAGE inhibitors in development, clinical trial enrichment and/or monitoring of patient response to therapy [8]. Previous efforts to develop an imaging agent for RAGE include 99mTc-labeled anti-RAGE antibodies, which showed promise in a mouse model of hind limb ischemia [9]. However, major limitations of antibody imaging agents are the long biological half-life and high background signal. Another biologic approach involved imaging with [18F]S100A4, a small protein ligand for RAGE, but did not prove useful as the labeled product was unstable and only had micromolar affinity for RAGE [10]. We recently developed [18F]RAGER [11], a small molecule radiotracer for RAGE based on the inhibitor FPS-ZM1 [12], and evaluated its properties for PET imaging of RAGE in the CNS. Independently, Kong et al.[13] and Bongarzone et al.[14] have also investigated FPS-ZM1 as a radiotracer for RAGE. Results across the three groups have been similar: blood-brain barrier permeability was confirmed in rodent and non-human primate, and increased binding was observed on post-mortem human brain tissue from dementia patients consistent with upregulation of RAGE in certain neurodegenerative disorders [11]. However, the present small molecule imaging efforts have suffered from high nonspecific binding and translation to clinical use has yet to be demonstrated.
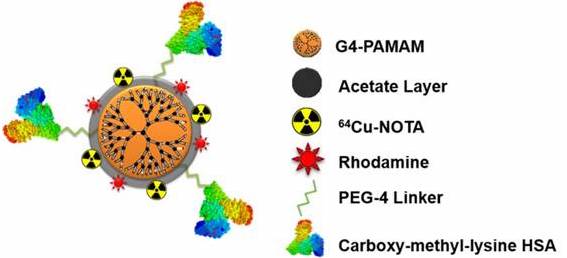
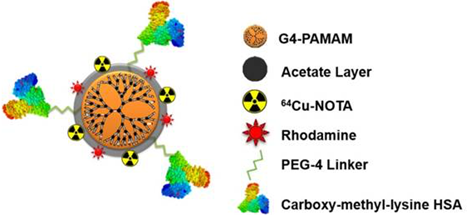
Recently, Konopka et al., reported a targeted nanoparticle approach for imaging RAGE [1]. Using the fourth generation PAMAM dendrimer and a NOTA chelator for fluorescence (rhodamine) or PET (copper-64) imaging, the authors developed a multimodal nanoparticle to target RAGE with the attached ligand, CML modified human serum albumin (Rho-G4-CML; Figure 1). Binding to RAGE was demonstrated in cellulo using HUVEC cells in a high glucose environment; calculated Kd= 340-390 nM. In vivo [64Cu]Rho-G4-CML uptake was evaluated in normal black mice for biodistribution and compared to the non-targeted [64Cu]Rho-G4-HSA nanoparticle, which showed both nanoparticles accumulate in the gall bladder, kidney, and intestines. Finally, the nanoparticle was evaluated in a mouse model of peripheral arterial damage (PAD): hindlimb ischemia in non-diabetic mice. In the model, PET imaging showed 3.4x higher uptake in hindlimbs of the ischemic mice compared to normal mice at the one week timepoint. PET imaging corresponded to an increase in RAGE expression, which is supported by previous immunohistochemistry analysis of this model [9]. At the two week timepoint, however, the increase in NP binding and RAGE expression had returned to basal levels. This time course of RAGE expression in hindlimb ischemia had previously only been demonstrated in genome sequencing studies [15].
A limitation of this study addressed by the authors is the non-specific binding concern. While not identified, the authors acknowledge that RAGE ligands (including CML) are not exclusive to this receptor. There is often cross-talk between RAGE and toll-like receptors, and these receptors can even heterodimerize and signal together. An investigation into other binding partners is surely required; in the HUVEC binding experiment the anti-RAGE competition only blocked 80% of nanoparticle binding. Nonspecific binding with RAGE ligands is not unique to this nanoparticle. In fact, with the small molecule [18F]RAGER, we have experienced high nonspecific binding to white matter, and this has complicated in vitro experiments [11]. Another curiosity with this study is the biodistribution experiment showing little to no lung uptake of the nanoparticle conjugate since high expression of RAGE in the lung is known [16]. Interestingly, [18F]S100A4 did not show high lung uptake in imaging studies either (1.5% injected dose/ g at 5 min and 0.5% at 60 min) [10]. Likewise, we undertook biodistribution experiments with [18F]RAGER in rodent that confirmed it does not accumulate in the lungs either [17], and this has been independently confirmed in mice [13]. The reasons for this discrepancy between the known expression of RAGE in lung, and the lack of appreciable uptake of any of these imaging agents are currently unclear but warrant further investigation.
Multimodal (PET-optical) RAGE-targeting 64Cu-Rho-G4-CML nanoparticle construct (Figure reproduced from [1]).
RAGE has been implicated in various diseases and disorders spanning from neurodegeneration, atherosclerosis, chronic obstructive pulmonary disease (COPD), diabetes, cancer, and others [2]. Is it a fading trend or a biomarker and/or therapeutic target with true potential? Given observed differences in disease to date, we support use of RAGE as a biomarker, but acknowledge the challenge of its promiscuity and splice isoforms. With a large variety of protein and peptide ligands for the extracellular domains, targeting the receptor with a small molecule has been difficult because there is no small ligand binding domain. The approach taken by Konopka et al. in this work with a CML-HSA coated nanoparticle is a successful use of a native ligand to target and image RAGE. Future theranostic applications of the nanoparticle conjugate for targeted drug delivery are also eluded to and could have utility in the development of RAGE-targeting drugs. However, the promiscuity of CML (and other ligands) requires further investigation into specificity and selectivity as soon as possible. Similarly, the approach to target the extracellular domains of RAGE does not offer selectivity over the various splice isoforms. Both full length, membrane bound RAGE and esRAGE share those domains, but have opposing functions. Recently, small molecule inhibitors of the intracellular domain of RAGE and the ligand DIAPH1 were discovered from high-throughput screening [18]. No therapeutic or imaging candidates have resulted from this work to date; however, we have been successful in labeling one of the intracellular inhibitors for preliminary evaluation [19]. While it is not fully understood if it would be more useful to image and inhibit RAGE or esRAGE, it is worth attempting to differentiate between these two isoforms when designing future imaging agents.
Acknowledgements
L.R.D is supported by the National Institutes of Health Pharmaceutical Sciences Training Program (T32-GM007767) and Rackham Graduate School at the University of Michigan.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Konopka C, Wozniak M, Hedhli J, Ploska A, Schwartz-Duval A, Siekierzycka A. et al. Multimodal imaging of the receptor for advanced glycation end-products with molecularly targeted nanoparticles. Theranostics. 2018;8:5012-24
2. Bongarzone S, Savickas V, Luzi F, Gee AD. Targeting the Receptor for Advanced Glycation Endproducts (RAGE): A Medicinal Chemistry Perspective. J Med Chem. 2017;60:7213-32
3. Kalea AZ, Schmidt AM, Hudson BI. Alternative splicing of RAGE: roles in biology and disease. Front Biosci. 2011;16:2756-70
4. Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R. et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238-43
5. Park L, Raman KG, Lee KJ, Lu Y, Ferran Jr LJ, Chow WS. et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025
6. Kalea AZ, See F, Harja E, Arriero M, Schmidt AM, Hudson BI. Alternatively Spliced RAGEv1 Inhibits Tumorigenesis through Suppression of JNK Signaling. Cancer Res. 2010;70:5628-38
7. Press Release. vTv Therapeutics Announces Topline Results from the First STEADFAST Phase 3 Study Evaluating Azeliragon in People with Mild Alzheimer's Disease. 2018: http://ir.vtvtherapeutics.com/phoenix.zhtml?c=254081&p=irol-newsArticle&ID=2341681, accessed 14-Nov-2018.
8. Matthews P, Rabiner E, Passchier J, Gunn R. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol. 2012;73:175-86
9. Tekabe Y, Kollaros M, Li C, Zhang G, Schmidt AM, Johnson L. Imaging receptor for advanced glycation end product expression in mouse model of hind limb ischemia. EJNMMI Res. 2013;3:37
10. Wolf S, Haase-Kohn C, Lenk J, Hoppmann S, Bergmann R, Steinbach J. et al. Expression, purification and fluorine-18 radiolabeling of recombinant S100A4: a potential probe for molecular imaging of receptor for advanced glycation endproducts in vivo? Amino Acids. 2011;41:809-20
11. Cary BP, Brooks AF, Fawaz MV, Drake LR, Desmond TJ, Sherman P. et al. Synthesis and Evaluation of [18F]RAGER: A First Generation Small-Molecule PET Radioligand Targeting the Receptor for Advanced Glycation Endproducts. ACS Chem Neurosci. 2016;7:391-8
12. Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B. et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377-92
13. Kong Y, Hua F, Guan Y, Zhao B. RAGE-specific probe 18F-FPS-ZM1 may be a promising biomarker for early detection of Diabetes with Alzheimer's disease. J Nucl Med. 2016;57(Suppl. 2):1049
14. Bongarzone S, Luzi F, Savickas V, Singh N, Turkheimer F, Gee AD. Development of a carbon-11 PET tracer for imaging the Receptor for Advanced GLycation Endproducts (RAGE) in Alzheimer's disease. J Label Compd Radiopharm. 2017;60(Suppl 1):140
15. Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K. et al. Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics. 2002;11:263-72
16. Brett J, Schmidt AM, Du Yan S, Zou YS, Weidman E, Pinsky D. et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699-712
17. Drake L, Brooks A, Scott P. Imaging the Receptor for Advanced Glycation Endproducts with [18F]RAGER. J Nucl Med. 2017;58(Suppl. 1):551
18. Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N. et al. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Sci Rep. 2016;6:22450
19. Drake L, Scott P. A Small Molecule Radioligand for the Intracellular Face of RAGE. J Nucl Med. 2018;59(Suppl. 1):1018
Author contact
![]() Corresponding author: pjhscottedu
Corresponding author: pjhscottedu
 Global reach, higher impact
Global reach, higher impact