13.3
Impact Factor
Theranostics 2018; 8(20):5610-5624. doi:10.7150/thno.27559 This issue Cite
Research Paper
Longitudinal intravital imaging of transplanted mesenchymal stem cells elucidates their functional integration and therapeutic potency in an animal model of interstitial cystitis/bladder pain syndrome
1. Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2. Department of Biomedical Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3. Department of Physiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
4. Department of Physics, Yonsei University, Seoul, Korea
5. Biomedical Engineering Research Center, ASAN Institute for Life Sciences, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea
6. Department of Convergence Medicine, University of Ulsan, College of Medicine, Seoul, Korea
7. Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea
8. Mirae Cell Bio Co. Ltd, Seoul, Korea
*These authors contributed equally to this work.
Abstract
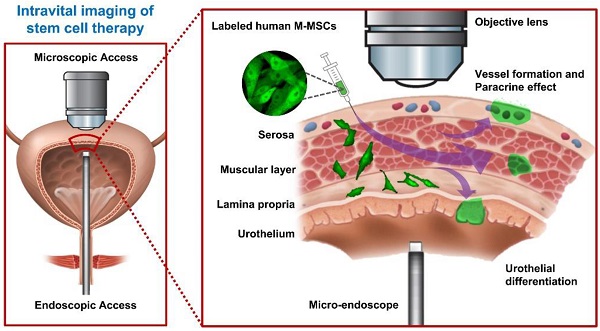
Rationale: Mesenchymal stem cell (MSC) therapy may be a novel approach to improve interstitial cystitis/bladder pain syndrome (IC/BPS), an intractable disease characterized by severe pelvic pain and urinary frequency. Unfortunately, the properties of transplanted stem cells have not been directly analyzed in vivo, which hampers elucidation of the therapeutic mechanisms of these cells and optimization of transplantation protocols. Here, we monitored the behaviors of multipotent stem cells (M-MSCs) derived from human embryonic stem cells (hESCs) in real time using a novel combination of in vivo confocal endoscopic and microscopic imaging and demonstrated their improved therapeutic potency in a chronic IC/BPS animal model.
Methods: Ten-week-old female Sprague-Dawley rats were instilled with 10 mg of protamine sulfate followed by 750 μg of lipopolysaccharide weekly for 5 weeks. The sham group was instilled with phosphate-buffered saline (PBS). Thereafter, the indicated dose (0.1, 0.25, 0.5, and 1×106 cells) of M-MSCs or PBS was injected once into the outer layer of the bladder. The distribution, perivascular integration, and therapeutic effects of M-MSCs were monitored by in vivo endoscopic and confocal microscopic imaging, awake cystometry, and histological and gene expression analyses.
Results: A novel combination of longitudinal intravital confocal fluorescence imaging and microcystoscopy in living animals, together with immunofluorescence analysis of bladder tissues, demonstrated that transplanted M-MSCs engrafted following differentiation into multiple cell types and gradually integrated into a perivascular-like structure until 30 days after transplantation. The beneficial effects of transplanted M-MSCs on bladder voiding function and the pathological characteristics of the bladder were efficient and long-lasting due to the stable engraftment of these cells.
Conclusion: This longitudinal bioimaging study of transplanted hESC-derived M-MSCs in living animals reveals their long-term functional integration, which underlies the improved therapeutic effects of these cells on IC/BPS.
Keywords: Intravital imaging, Multipotent stem cell, Mesenchymal stem cell, Embryonic stem cell, Interstitial cystitis/bladder pain syndrome.
 Global reach, higher impact
Global reach, higher impact