13.3
Impact Factor
Theranostics 2018; 8(18):5126-5142. doi:10.7150/thno.27221 This issue Cite
Research Paper
Ultrasound molecular imaging as a non-invasive companion diagnostic for netrin-1 interference therapy in breast cancer
1. Univ Lyon, Université Lyon 1, Centre Léon Bérard, INSERM, LabTAU, F-69003, LYON, France.
2. Department of Radiology, School of Medicine, Stanford University, 94305 Stanford, USA.
3. Apoptosis, Cancer and Development Laboratory - Equipe labellisée 'La Ligue', LabEx DEVweCAN, Centre de Cancérologie de Lyon, INSERM U1052-CNRS UMR5286, Centre Léon Bérard, 69008 Lyon, France ;
4. Netris Pharma, 69008 Lyon, France.
*P. Mehlen, J. K. Willmann and F. Padilla contributed equally as senior authors of this article.
†deceased on January 8, 2018
Abstract
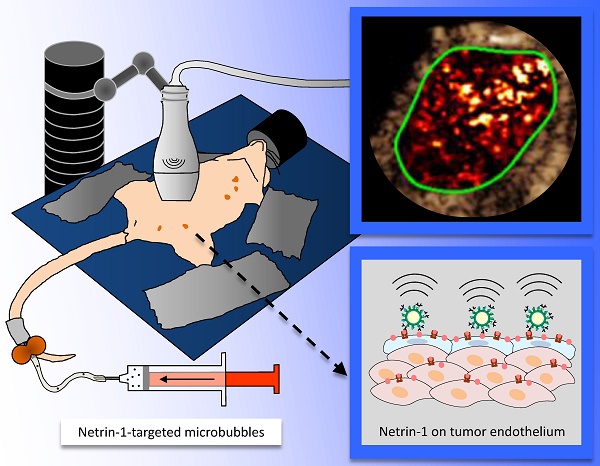
In ultrasound molecular imaging (USMI), ligand-functionalized microbubbles (MBs) are used to visualize vascular endothelial targets. Netrin-1 is upregulated in 60% of metastatic breast cancers and promotes tumor progression. A novel netrin-1 interference therapy requires the assessment of netrin-1 expression prior to treatment. In this study, we studied netrin-1 as a target for USMI and its potential as a companion diagnostic in breast cancer models.
Methods: To verify netrin-1 expression and localization, an in vivo immuno-localization approach was applied, in which anti-netrin-1 antibody was injected into living mice 24 h before tumor collection, and revealed with secondary fluorescent antibody for immunofluorescence analysis. Netrin-1 interactions with the cell surface were studied by flow cytometry. Netrin-1-targeted MBs were prepared using MicroMarker Target-Ready (VisualSonics), and validated in in vitro binding assays in static conditions or in a flow chamber using purified netrin-1 protein or netrin-1-expressing cancer cells. In vivo USMI of netrin-1 was validated in nude mice bearing human netrin-1-positive SKBR7 tumors or weakly netrin-1-expressing MDA-MB-231 tumors using the Vevo 2100 small animal imaging device (VisualSonics). USMI feasibility was further tested in transgenic murine FVB/N Tg(MMTV/PyMT634Mul) (MMTV-PyMT) mammary tumors.
Results: Netrin-1 co-localized with endothelial CD31 in netrin-1-positive breast tumors. Netrin-1 binding to the surface of endothelial HUVEC and cancer cells was partially mediated by heparan sulfate proteoglycans. MBs targeted with humanized monoclonal anti-netrin-1 antibody bound to netrin-1-expressing cancer cells in static and dynamic conditions. USMI signal was significantly increased with anti-netrin-1 MBs in human SKBR7 breast tumors and transgenic murine MMTV-PyMT mammary tumors compared to signals recorded with either isotype control MBs or after blocking of netrin-1 with humanized monoclonal anti-netrin-1 antibody. In weakly netrin-1-expressing human tumors and normal mammary glands, no difference in imaging signal was observed with anti-netrin-1- and isotype control MBs. Ex vivo analysis confirmed netrin-1 expression in MMTV-PyMT tumors.
Conclusions: These results show that USMI allowed reliable detection of netrin-1 on the endothelium of netrin-1-positive human and murine tumors. Significant differences in USMI signal for netrin-1 reflected the significant differences in netrin-1 mRNA & protein expression observed between different breast tumor models. The imaging approach was non-invasive and safe, and provided the netrin-1 expression status in near real-time. Thus, USMI of netrin-1 has the potential to become a companion diagnostic for the stratification of patients for netrin-1 interference therapy in future clinical trials.
Keywords: ultrasound molecular imaging, netrin-1, companion diagnostic, breast cancer, targeted microbubbles
 Global reach, higher impact
Global reach, higher impact