13.3
Impact Factor
Theranostics 2018; 8(11):3126-3137. doi:10.7150/thno.24784 This issue Cite
Research Paper
Image-guided chemotherapy with specifically tuned blood brain barrier permeability in glioma margins
1. Minhang Branch, Zhongshan Hospital and Key Laboratory of Smart Drug Delivery, Ministry of Education, School of Pharmacy, Fudan University, Shanghai, China.
2. Department of Neurosurgery, Huashan Hospital, Fudan University, Shanghai, China.
3. Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA, USA
4. Department of Pancreatic and Hepatobiliary Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
5. National Center for Magnetic Resonance in Wuhan, State Key laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, Wuhan, Hubei Province, China
6. Institute of Functional and Molecular Medical Imaging, Department of Radiology, Huashan Hospital, Fudan University, Shanghai, China.
7. Department of Laboratory Medicine, Shanghai Fengxian District Central Hospital, Shanghai, China.
*These authors share equal first authorship.
Abstract
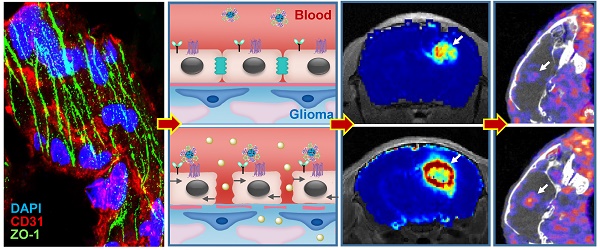
Blood-brain barrier (BBB) disruption is frequently observed in the glioma region. However, the tumor uptake of drugs is still too low to meet the threshold of therapeutic purpose.
Method: A tumor vasculature-targeted nanoagonist was developed. Glioma targeting specificity of the nanoagonist was evaluated by in vivo optical imaging. BBB permeability at the glioma margin was quantitatively measured by dynamic contrast enhanced magnetic resonance imaging (DCE-MRI). Single-photon emission computed tomography imaging/computed tomography (SPECT/CT) quantitatively determined the glioma uptake of the radiolabeled model drug. T2-weighted MRI monitored the tumor volume.
Results: Immunostaining studies demonstrated that the BBB remained partially intact in the invasive margin of patients' gliomas regardless of their malignancies. DCE-MRI showed that vascular permeability in the glioma margin reached its maximum at 45 min post nanoagonist administration. In vivo optical imaging indicated the high glioma targeting specificity of the nanoagonist. SPECT/CT showed the significantly enhanced glioma uptake of the model drug after pre-treatment with the nanoagonist. Image-guided paclitaxel injection after nanoagonist-mediated BBB modulation more efficiently attenuated tumor growth and extended survival than in animal models treated with paclitaxel or temozolomide alone.
Conclusion: Thus, image-guided drug delivery following BBB permeability modulation holds promise to enhance the efficacy of chemotherapeutics to glioma.
Keywords: image-guided chemotherapy, dynamic contrast-enhanced magnetic resonance imaging, blood-brain-barrier, glioma margin, nanoagonist
 Global reach, higher impact
Global reach, higher impact