13.3
Impact Factor
Theranostics 2018; 8(3):767-784. doi:10.7150/thno.21209 This issue Cite
Research Paper
A Tumor-Activatable Theranostic Nanomedicine Platform for NIR Fluorescence-Guided Surgery and Combinatorial Phototherapy
1. Department of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Portland, OR 97201, United States;
2. Department of Clinical Sciences, College of Veterinary Medicine, Oregon State University, Corvallis, OR 97331, United States.
Abstract
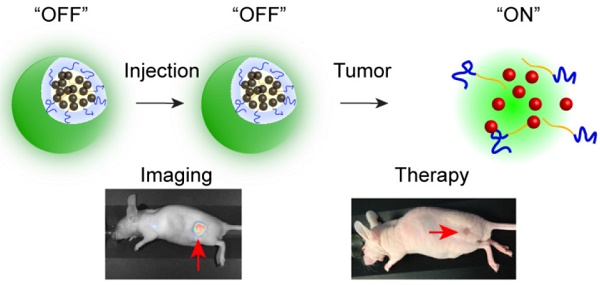
Fluorescence image-guided surgery combined with intraoperative therapeutic modalities has great potential for intraoperative detection of oncologic targets and eradication of unresectable cancer residues. Therefore, we have developed an activatable theranostic nanoplatform that can be used concurrently for two purposes: (1) tumor delineation with real-time near infrared (NIR) fluorescence signal during surgery, and (2) intraoperative targeted treatment to further eliminate unresected disease sites by non-toxic phototherapy.
Methods: The developed nanoplatform is based on a single agent, silicon naphthalocyanine (SiNc), encapsulated in biodegradable PEG-PCL (poly (ethylene glycol)-b-poly(ɛ-caprolactone)) nanoparticles. It is engineered to be non-fluorescent initially via dense SiNc packing within the nanoparticle's hydrophobic core, with NIR fluorescence activation after accumulation at the tumor site. The activatable nanoplatform was evaluated in vitro and in two different murine cancer models, including an ovarian intraperitoneal metastasis-mimicking model. Furthermore, fluorescence image-guided surgery mediated by this nanoplatform was performed on the employed animal models using a Fluobeam® 800 imaging system. Finally, the phototherapeutic efficacy of the developed nanoplatform was demonstrated in vivo.
Results: Our in vitro data suggest that the intracellular environment of cancer cells is capable of compromising the integrity of self-assembled nanoparticles and thus causes disruption of the tight dye packing inside the hydrophobic cores and activation of the NIR fluorescence. Animal studies demonstrated accumulation of activatable nanoparticles at the tumor site following systemic administration, as well as release and fluorescence recovery of SiNc from the polymeric carrier. It was also validated that the developed nanoparticles are compatible with the intraoperative imaging system Fluobeam® 800, and nanoparticle-mediated image-guided surgery provides successful resection of cancer tumors. Finally, in vivo studies revealed that combinatorial phototherapy mediated by the nanoparticles could efficiently eradicate chemoresistant ovarian cancer tumors.
Conclusion: The revealed properties of the activatable nanoplatform make it highly promising for further application in clinical image-guided surgery and combined phototherapy, facilitating a potential translation to clinical studies.
Keywords: NIR theranostic, activatable, silicon naphthalocyanine, PEG-PCL, ovarian cancer.
 Global reach, higher impact
Global reach, higher impact