13.3
Impact Factor
Theranostics 2017; 7(4):952-961. doi:10.7150/thno.16647 This issue Cite
Research Paper
Antigen-responsive molecular sensor enables real-time tumor-specific imaging
1. Molecular Imaging and Therapy Branch, National Cancer Center, 323 Ilsan-ro, Goyang, Gyeonggi 10408, Republic of Korea.
2. Gordon Center for Medical Imaging, Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
3. Biomolecular Function Research Branch, National Cancer Center, 323 Ilsan-ro, Goyang, Gyeonggi 10408, Republic of Korea.
Received 2016-6-29; Accepted 2017-1-2; Published 2017-2-23
Abstract
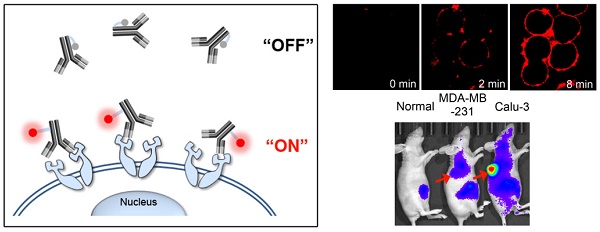
Antibody-fluorophore conjugates have high potential for the specific fluorescence detection of target cancer cells in vitro and in vivo. However, the antibody-fluorophore conjugates described to date are inappropriate for real-time imaging of target cells because removal of unbound antibody is required to reduce background fluorescence before quantifiable analysis by microscopy. In addition, clinical applications of the conjugates have been limited by persistent background retention due to their long systemic circulation and nonspecific uptake. Here we report fast and real-time near-infrared fluorescence imaging of target cancer cells using an antigen-responsive molecular “on-off” sensor: the fluorescence of trastuzumab-ATTO680 conjugate is dark (i.e., turned off) in the extracellular region, while it becomes highly fluorescent (i.e., turned on) upon binding to the target antigen HER2 on cancer cell surface. This molecular switch enables fast and real-time imaging of target cancer cells in vitro and in vivo.
Keywords: Antigen-responsive, near-infrared fluorescence imaging, real-time imaging.
Introduction
Detection of localized and disseminated tumor cells in patients is of significant importance for the success of cancer surgery and improving the survival rates of patients [1-3]. For example, minimizing the residual tumor size after cytoreductive surgery is known to be the most important factor for improving the survival rate of ovarian cancer patients [4, 5]. However, real-time discrimination of tumor and tumor margins from surrounding normal tissues has been a major challenge for surgeons during surgical procedures, even though conventional medical imaging modalities are routinely used prior to and/or during surgery [6]. Therefore, many efforts have been focused on the complete resection of both gross and microscopic tumors while preventing the loss of healthy normal tissues by precisely defining the tumor boundaries [7].
Near-infrared (NIR) fluorescence imaging has been used for real-time detection of cancers during open surgery and endoscopic procedures [6-8]. Compared with other medical imaging modalities, NIR fluorescence imaging has many advantages including high sensitivity and spatial resolution, non-contact imaging, simple procedure, low cost, and detection without ionizing irradiation, which enable real-time image-guided navigation [7]. Despite recent advances in intraoperative fluorescence imaging systems, the lack of targeted contrast agents has always been the major challenge in clinical translation.
Armed with the specific interaction of an antibody to the target cancer cells, antibody-fluorophore conjugates have shown great potential in the clinic [9, 10]. When a conventional fluorescence probe (i.e., “always-on”) is conjugated on a targeted antibody, however, it continuously emits photons regardless of its proximity or binding with target cancer cells, which sometimes limit the use of bioconjugates for specific delineation of tumorous tissues [11, 12]. In addition, the nonspecific uptake in major organs and long-term background retention due to the relatively long half-life (typically 1-3 weeks) of bioconjugates limit their specificity in tumors (and thus the tumor-to-background ratio; TBR) [12-14]. Rapid renal clearance of bioconjugates using zwitterionic fluorophores [15] or smaller antibody fragments [10] can eliminate the background retention issue, which generally requires high doses to accumulate the short circulating conjugates in the target tumors.
To overcome the limitations of using "always-on" type probes, activatable antibody-fluorophore conjugates have recently been developed [11, 12]. In this design, fluorescence signals of antibody-fluorophore conjugates quench by fluorescence resonance energy transfer (FRET) among the conjugated NIR fluorophores. When the antibody conjugates internalize into the cancer cells, the conjugated fluorophores release from the antibody by proteolytic degradation within the intracellular lysosome and turn the fluorescence on by the loss of FRET. These FRET-based activatable systems, however, require a relatively long time for receptor-mediated endocytosis as well as lysosomal degradation of the conjugates inside the cells [16, 17], which limit real-time intraoperative tumor imaging.
In addition, Antibody-fluorophore conjugates have also been widely used for specific fluorescence detection of cell surface antigens in the in vitro cell studies. However, antibody-fluorophore conjugates developed to date are impractical for live-cell fluorescence imaging studies or in situ detection of moving cells in microfluidic device systems because of nonspecific uptake and persistent washout period for imaging. Therefore it is highly challenging to develop antibody-based molecular sensors which enable fast and real-time imaging of target cells.
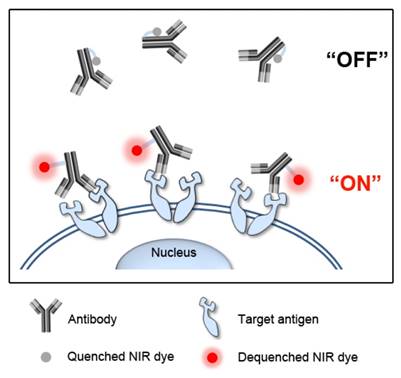
Here, we developed antigen-responsive antibody-fluorophore conjugates for fast, specific, and real-time fluorescence imaging of target cancer cells in vitro and in vivo. As shown in Figure 1, the NIR fluorophores conjugated to tryptophan (Trp)-rich antibodies show no fluorescence outside cells (i.e., turn-off) by the photoinduced electron transfer (PET) with nearby Trp residues [18, 19]. When the antibody conjugates reach the target antigens on cancer cell surface, the 3D conformational changes eliminate the hydrophobic interactions and move fluorophores far from the Trp residues, resulting in immediate dequenching (i.e., turn-on) of fluorescence. This antigen-responsive molecular sensor enabled real-time fluorescence imaging of cancer cells in vitro and in vivo with a high target-to-background ratio.
Schematic diagram of antigen-responsive antibody-fluorophore conjugates for activatable fluorescence imaging of cancer cells. NIR fluorescence of the conjugates stays off at normal tissues and in the extracellular region, but becomes highly fluorescent immediately after binding to the target antigens overexpressed on the cancer cell surface.
Materials and Methods
Materials
Herceptin® (trastuzumab), a Food and Drug Administration-approved humanized anti-HER2 IgG1 monoclonal antibody, was obtained from Roche Pharma (Basel, Switzerland). Amine-reactive ATTO655-N-hydroxysuccinimidyl ester (ATTO655-NHS ester; λex/λem: 663/684), ATTO680-NHS ester (λex/λem: 680/700), ATTO700-NHS ester (λex/λem: 700/719), and free ATTO680-COOH were purchased from ATTO TEC (Siegen, Germany). NHS-Fluorescein (λex/λem: 494/518), Alexa Fluor® 488-NHS ester (λex/λem: 494/518), and Alexa Fluor® 680-NHS ester (λex/λem: 679/702) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). L-Trptophan and L-methionine were purchased from Sigma-Aldrich (St. Louis, MO, USA). N-Acetyl-L-Tyrosine and L-histidine were purchased from Tokyo Chemical Industry (Tokyo, Japan).
Synthesis of trastuzumab-fluorophore conjugates
Trastuzumab (0.5 mg, 3.4 nmol) and amine-reactive fluorophores (antibody: dye mole ratio = 1:1, 1:5, 1:10, 1:15) were simultaneously dissolved in 0.3 mL phosphate-buffered saline (PBS, pH 7.4, 10 mM, NaCl, 137 mM) at room temperature and reacted for 1 h under light-protected conditions with gentle shaking. Unbound fluorophores and byproducts were removed from the mixture using a Sephadex G25 gel filtration column (PD-10; GE Healthcare, Little Chalfont, UK). Next, purified trastuzumab-fluorophore conjugates were concentrated using Amicon Ultra-0.5 mL centrifugal filters (Membrane cut off: 50 K, EMD Millipore, Billerica, MA, USA) and stored at 4°C for use as a stock solution.
Characterization of trastuzumab-fluorophore conjugates
The average number of fluorophores conjugated to trastuzumab (i.e., degree of labeling, DL) was determined by measuring the absorbance of trastuzumab-fluorophore conjugates in PBS using an UV/Vis spectrophotometer (DU750, Beckman Coulter, Brea, CA, USA). To measure the trastuzumab concentration, the molar extinction coefficient of IgG (i.e., 210,000 M-1cm-1 at 280 nm) was used. Molar extinction coefficients for the fluorophores were obtained from the manufacturer website. To analyze the fluorescence quenching ability in vitro, fluorophores and trastuzumab-fluorophore conjugates were diluted in PBS to obtain final concentration of 1 μM dye equivalent, and then the fluorescence spectra and intensities were recorded on a fluorescence microplate reader (Safire II, Tecan, Durham, NC, USA). The fluorescence intensities of free fluorophores at 1 μM were set to zero DL.
Quenching and dequenching properties of trastuzumab-ATTO680
The trastuzumab-ATTO680 conjugate with 3.77 DL (HER-ATTO680) showed the best quenching effect with good dispersion in PBS (pH 7.4); thus, we used this conjugate in subsequent analyses. To denature HER-ATTO680, a stock solution of HER-ATTO680 was diluted with PBS containing 1 % SDS and 1 mM 2-mercaptoethanol, and then heated at 95°C for 2 min. Denaturing buffer and the denaturation process did not affect the optical properties of ATTO680 dye (data not shown). The fluorescence spectra of the samples were obtained using a fluorescence microplate reader (λex 620 nm, λem 640-850 nm). The fluorescence spectrum of PBS without ATTO680 dye was measured as a control. NIR fluorescence images of free ATTO680-COOH, HER-ATTO680, and denatured HER-ATTO680 at 5 μM dye equivalent were obtained with IVIS Lumina Imaging System (Xenogen, Alameda, CA, USA, λex 660/20 nm, λem 710/40 nm) to visualize the fluorescence quenching of the conjugate. PBS was used as a control.
Quenching of free ATTO680 dye
Various concentrations of tryptophan, tyrosine, methionine, and histidine were prepared by diluting the stock solutions with deionized water. Since tyrosine has limited water solubility, highly water-soluble N-acetyl-L-tyrosine was used. Free ATTO680 was dissolved in the amino acid solutions at a final concentration of 1 μM and then fluorescence intensities were measured (λex 680 nm, λem 700 nm) using a microplate reader (Safire II, Tecan, Männedorf, Switzerland).
Cell culture
The Calu-3 (human lung adenocarcinoma) and SK-BR-3 (human breast adenocarcinoma) cell lines were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). The KPL4 (human breast cancer) cell line was kindly provided by Dr. Junichi Kurebayashi (Kawasaki Medical Hospital, Kurashiki, Okayama, Japan) [20]. The MDA-MB-231 (human breast adenocarcinoma) cell line was purchased from Perkin Elmer (Waltham, MA, USA). SK-BR-3, KPL4, and MDA-MB-231 cells were grown in RPMI-1640 medium (Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific Inc.) and 1% Antibiotic & Antimycotic ( Thermo Fisher Scientific Inc.) in a humidified atmosphere containing 5% CO2. Calu-3 cells were grown in Eagle's Minimum Essential Medium (EMEM, ATCC) supplemented with 10% fetal bovine serum and 1% Antibiotic & Antimycotic in a humidified atmosphere containing 5% CO2.
Evaluation of HER2-targeting specificity of HER-ATTO680
SK-BR-3, KPL4, and Calu-3 are HER2-positive cell lines, whereas MDA-MB-231 is a HER2-negative cell line. Each cell line was plated at a density of 5 × 104/well on a 4-well Lab-Tek II chambered coverglass (Thermo Fisher Scientific Inc.) and incubated overnight for cell attachment. Next, HER-ATTO680 was added to the medium (20 μg/mL), and the cells were incubated for either 30 min or 1 h at 37ºC. The cells were washed twice with PBS solution, and phenol red-free cell culture medium was added. Confocal fluorescence images (λex 633 nm, λem 647-754 nm) were acquired using a confocal laser scanning microscope (CLSM, Carl Zeiss LSM510 META, Jena, Germany) with a 40X water immersion objective lens.
To investigate the targeting specificity further, a blocking study was performed by adding unlabeled trastuzumab before HER-ATTO680 treatment. HER2-positive SK-BR-3 and Calu-3 cell lines were plated at a density of 5 x 104 cells/well on a 4-well Lab-Tek II chambered coverglass and incubated overnight for cell attachment. The cell lines were pre-treated with Trastuzumab (100 μg) for 1 h; then HER-ATTO680 (20 μg/mL) was added and incubated for 30 min. After washing the cells, confocal fluorescence images were acquired (λex 633 nm, λem 647-754 nm).
Analysis of nonspecific uptake of free dye
HER2-positive SK-BR-3 cells were plated at a density of 5 × 104/well on a 4-well Lab-Tek II chambered coverglass, and incubated overnight for cell attachment. Free ATTO680-COOH was dissolved and diluted in phenol-free cell culture medium to a final concentration of 1 μM. Next, the cell culture medium was replaced with phenol-free medium containing free ATTO680-COOH at 1 μM and incubated for 30 min at 37°C. Confocal fluorescence images were obtained before and after washing the cells. Untreated cells were used as a control.
Fluorescence recovery upon cell binding
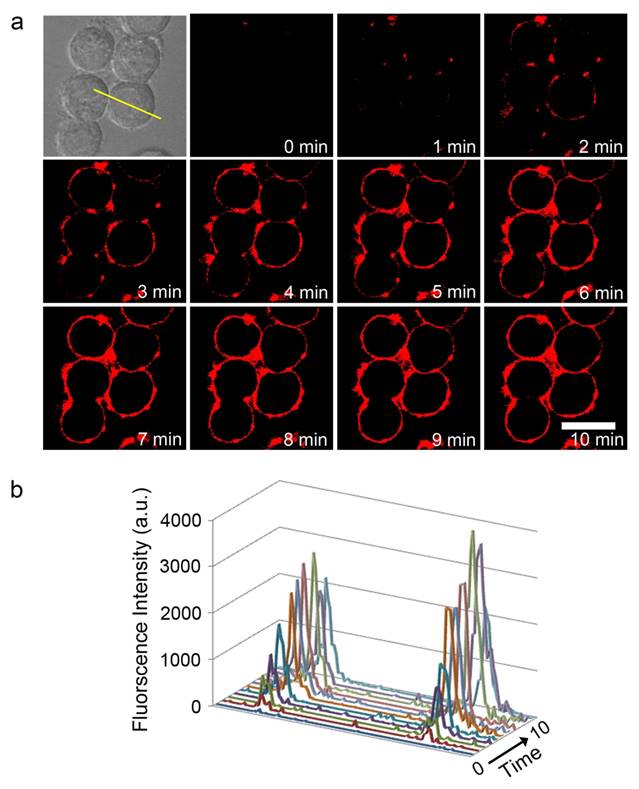
SK-BR-3 cells were plated at a density of 5 × 104/well on a 4-well Lab-Tek II chambered coverglass and incubated overnight for cell attachment. Next, HER-ATTO680 was added to the medium (10 μg/mL). To demonstrate HER2-responsive fluorescence turn-on during real-time fluorescence imaging, confocal fluorescence images (λex 633 nm, λem 647-754 nm) were acquired every 1 min for 10 min in a selected area without washing the cells. Confocal images were analyzed using the LSM 5 image browser, and profiling was conducted using Axiovision software (Carl Zeiss).
Kinetics of intracellular uptake of bound HER-ATTO680
SK-BR-3 cells were plated at a density of 5 × 104/well on a 4-well Lab-Tek II chambered coverglass and incubated overnight for cell attachment. Next, the cells were treated with cell culture medium containing HER-ATTO680 (20 μg/mL) for 30 min, washed three times, and further incubated for 24 h. Confocal fluorescence images (λex 633 nm, λem 647-754 nm) were acquired at 30 min, 1 h, 5 h, and 24 h. Thirty minutes before imaging, lysosomes in the cells were counterstained with Lysotracker Blue DND-22 (Thermo Fisher Scientific Inc.) for comparison.
In vivo studies in xenograft tumor models
All animal studies were approved by the Institutional Animal Care and Use Committee. Female athymic nude mice (Balb/c-nu, 5 weeks olds) were used for in vivo experiments. Calu-3 and MDA-MB-231 cells (5 × 106 cells/0.1 mL serum-free medium) were implanted subcutaneously into the right hind flank of each mouse, and tumor size was measured daily [21]. A total of 15 mice were used for in vivo imaging analysis when tumor sizes reached ~190 mm3. For in vivo NIR fluorescence imaging, six mice with Calu-3 and MDA-MB-231 tumors received intravenous injections of the HER-ATTO680 (50 µg conjugate/50 µL PBS/mouse). Three mice in the control group with no tumors received intravenous injections of 50 µL PBS. NIR fluorescence images were obtained using the IVIS Lumina imaging system (λex = 660/20 nm, λem = 710/40 nm) at 1, 5, and 24 h after injection. To investigate the biodistribution of the conjugate, additional six mice with Calu-3 and MDA-MB-231 tumors received intravenous injections of the HER-ATTO680 (50 µg conjugate/50 µL PBS/mouse). At 1, 5, and 24 h post-injection, the mice were sacrificed and NIR fluorescence images of the tumor, kidney, spleen, and liver (λex = 660/20 nm, λem = 710/40 nm) were obtained. Then, the tumor tissues collected were immersed in OCT solution, and sectioned at 7 μm thickness. After staining the nuclei of the tumor cells with mounting media solution containing DAPI (Vector Laboratories, Inc., Burlingame, CA, USA), fluorescence images of tumor tissues were observed using a confocal scanning laser microscope (Carl Zeiss LSM 780, λex 633 nm, and λem 647-754 nm for HER-ATTO680; λex = 405 nm, and λem 420-480 nm for DAPI).
Results and Discussion
Trastuzumab conjugation with fluorophores
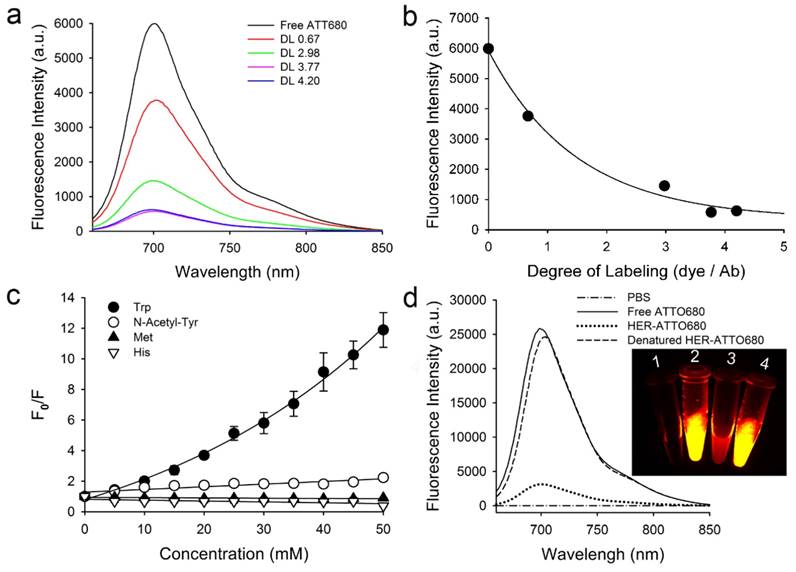
We selected trastuzumab, a humanized monoclonal antibody, for tumor targeting because of its high binding affinity to the extracellular domain of human epidermal growth factor receptor 2 (HER2) in many cancers [22,23]. Figure S1 shows the 3D structure of trastuzumab with the marked positions of Trp, tyrosine (Tyr), and lysine (Lys). A series of fluorophores such as fluorescein, Alexa488, Alexa680, ATTO655, ATTO680, and ATTO700 were reacted with the Lys residues of trastuzumab at various molar ratios for conjugation. Most of these fluorophores undergo fluorescence quenching by Trp and/or Tyr by the PET mechanism [18,19]. After conjugation and purification, the degree of labeling (DL) between the antibody and conjugated dyes was calculated by measuring absorbance spectra at 280 nm for antibody and at λabs for each dye. As shown in Figure 2 and Figure S2 in the Supporting Information, the fluorescent signals decreased proportionally with increasing the DL, resulted from the fluorescence quenching by PET. Especially, HER-ATTO680 with a DL of 3.77 showed the highest fluorescence quenching among the tested (i.e., 7.9-fold inhibition in the NIR window; Figure 2a and Figure 2b), and therefore we used HER-ATTO680 for the rest of experiments. Figure 2c and S3 shows that free ATTO680 dyes highly interact with Trp (i.e., F0/F = 12 at 50 mM Trp) as well as with Tyr (i.e., F0/F = 2.2 at 50 mM Tyr) [18]. NIR fluorescence spectra and images of phosphate-buffered saline (PBS, pH 7.4), free ATTO680, HER-ATTO680, and HER-ATTO680 treated with denatured buffer solution (1% SDS, 1 mM 2-mercaptoethanol) are shown in Figure 2d for comparison.
In vitro studies and live-cell imaging in cancer cells
Prior to in vitro cell study, we evaluated the stability of HER-ATTO680 in serum (Figure S4). The conjugate showed no notable increase in fluorescence over the 24 h incubation period in both PBS and serum, which indicates that no dyes were cleaved from the conjugates, and thus, the quenched form of HER-ATTO680 is highly stable at the physiological condition. In addition, HER-ATTO 680 was shown to be stable when incubated at physiological (pH 7.4) and lysosomal (pH 5.0) pH conditions at least for 16 h (Figure S5).
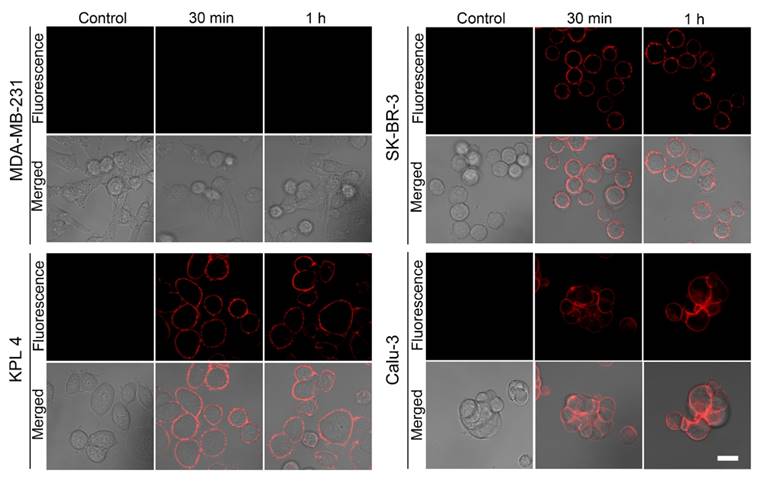
Next, the binding specificity was evaluated by treating HER-ATTO680 in HER2-positive (SK-BR-3, KPL4, and Calu-3) and HER2-negative (MDA-MB-231) cancer cell lines for 30 min or 1 h. NIR fluorescence images (λex 633 nm, λem 647-754 nm) were obtained by confocal fluorescence microscopy after washing the plate 3 times with incubating media. As shown in Figure 3, strong fluorescence signals were detected on the surface of the HER2-positive cancer cells, whereas no detectable fluorescence was in the HER2-negative cancer cells. When HER2-positive cells were pre-incubated with unlabeled trastuzumab before HER-ATTO680 treatment, no fluorescence signals were observed on the surface of the cells (Figure S6). These data confirm the binding specificity of HER-ATTO680 to the target antigen HER2.
Characterization of ATTO680-conjugated trastuzumab (HER-ATTO680). a) Fluorescence spectra (λex 630 nm, λem 640-850 nm) of free ATTO680 (1 μM) and trastuzumab-ATTO680 conjugates (1 μM eq.) at various degrees of labeling (DL). b) Fluorescence intensities (λex 620 nm, λem 700 nm) vs. DL of HER-ATTO680 conjugates. c) Fluorescence quenching of free ATTO680 dye by amino acids. Fluorescence intensities of free ATTO680 were measured in the presence (F) and absence (F0) of amino acids at various concentrations (n = 3; N-Acetyl-L-Tyr = N-acetylated Tyr). d) Fluorescence spectra of free dye, HER-ATTO680, and denatured HER-ATTO680 at 5 μM dye equivalent. Inset images represent NIR fluorescence imaging (λex 660/20 nm, λem 710/40 nm) of the sample tubes containing: 1) PBS solution, 2) free ATTO680-COOH, 3) HER-ATTO680, and 4) denatured HER-ATTO680.
Antigen-responsive binding of HER-ATTO680 at the surface of HER2-positive cancer cells. HER2-negative (MDA-MB-231) and HER2-positive (SK-BR-3, KPL4, and Calu-3) cells were treated with 20 μg/mL HER-ATTO680 for 30 min and 1 h, respectively. The confocal fluorescence microscopy images (λex 633 nm, λem 647-754 nm) of the cells were acquired after washing the cells with cell culture media. Cells in the control groups were treated with cell culture media only, and their NIR fluorescence images were obtained for comparison. Scale bar = 20 μm.
To demonstrate the potential utility of HER-ATTO680 for rapid and real-time fluorescence imaging, HER2-positive cancer cells were imaged every 1 min after adding HER-ATTO680 by using confocal microscopy (λex 633 nm, λem 647-754 nm). As shown in Figure 4, strong fluorescence signals were observed along the cancer cell membrane beginning at 2 min post-treatment, indicating that HER-ATTO680 bound to the cell surface antigens and turned its fluorescence on. The signals on the cell surface increased over the time course of incubation and saturated around 8 min post-incubation (Figure 4b), resulting in more than 100-fold higher fluorescence intensity on the cancer cell surface compared with that of the surrounding background noise from the extracellular space. For comparison, SK-BR-3 cells were incubated with free ATTO680 dye (Figure S7) and fluorescence images were obtained after 30 min of incubation. Strong fluorescence signals from the “always on” dye were detected in the extracellular space of the free dye-treated cells. After washing the cells with cell culture media, however, almost no fluorescence was observed by the confocal microscopy, indicating that free ATTO680 dyes were washed out without specific binding on the cell surface.
To evaluate fluorescence turn-on of HER-ATTO 680 upon binding to the target cells further, trastuzumab-Alexa647 conjugate with 3.63 DL (i.e., HER-Alexa647) was synthesized as an “always-on” type of antibody-fluorophore conjugate for comparison (see Supporting information for details). Alexa 647 dye did not undergo fluorescence quenching in the presence of Trp or Tyr. In addition, no significant fluorescence quenching of HER-Alexa 647 was observed at this DL value (data not shown here). HER2-postive SK-BR-3 cells were incubated with HER-Alexa647 for 30 min and confocal fluorescence images (λex 633 nm, λem 647-754 nm) were acquired without washing the cells. High fluorescence background signals were generated in the extracellular region (Figure S8a).
Next, we measured the rate of receptor-mediated endocytosis of the bound trastuzumab conjugate in HER2-positive SK-BR-3 cells. The cells were incubated with HER-ATTO680 for 30 min followed by washing three times with cell culture media to remove unbound conjugates. As shown in Figure S9a, most conjugates remained on the cell surface up to 5 h post-incubation, and a large portion of the membrane signals stayed even 24 h post-treatment, indicating slow internalization of bound trastuzumab conjugates into SK-BR-3 cells. Therefore, antigen-responsive HER-ATTO680 is applicable for fast and real-time NIR fluorescence imaging of cancer cells, regardless of the endocytosis process. In addition, quantitative analysis revealed that the fluorescence intensity of HER-ATTO680 in the lysosomes of SK-BR-3 cells was not significantly higher than that on the cell surface at 30 min and 24 h after treatment (Figure S9b). According to Kobayashi et al. [11, 12], an antibody-fluorophore conjugate with fluorescence resonance energy transfer (FRET)-based self-quenching behavior showed significant fluorescence increase in the lysosomal sites of the target cancer cells because enzymatic degradation of the antibody-fluorophore conjugate in the lysosomes resulted in the release of conjugated dyes from the antibody and subsequent removal of FRET effect. The result shown in Figure S9b also support that HER-ATTO680 was quenched by PET-based mechanism and mostly dequenched upon binding with target antigens on the cell surface; thus, no further fold-increase in fluorescence intensity occurred in the lysosomal sites.
Real-time fluorescence imaging of membrane binding HER-ATTO680 on SK-BR-3 cells. a) Time-lapse confocal fluorescence images of HER-ATTO680 in SK-BR-3 cells were acquired every 1 min (λex 633 nm, λem 647-754 nm) without washing the cells, to prove the switch on of fluorescence signals upon membrane binding. b) Fluorescence intensity across the area indicated by the yellow line in the bright-field image of a) was analyzed over time. Scale bar = 20 μm.
In vivo and ex vivo imaging in xenograft models
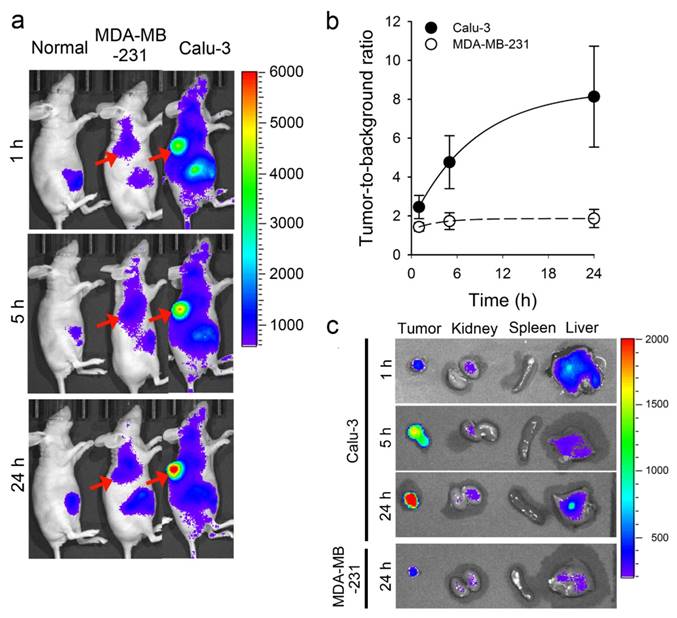
We, then, evaluated the potential utility of HER-ATTO680 for antigen-responsive specific imaging in tumor animal models. HER2-positive Calu-3 and HER2-negative MDA-MB-231 cells were separately implanted into the frank of Balb/c-nu mice. When tumor sizes reached around 190 mm3, the tumor mice were injected intravenously with 50 μg of HER-ATTO680 in 50 μL PBS. Balb/c-nu mice without tumor implantation were included as control for comparison and received 50 μL of PBS. NIR fluorescence images of all mice were obtained using an IVIS Lumina imaging system (λex = 660/20 nm, λem = 710/40 nm) at 1, 5, and 24 h post-injection and their TBR was analyzed. According to Figure 5a and Figure S10, the tumor sites of Calu-3-bearing mice could be discriminated from the NIR fluorescence images from 1 h post-injection of HER-ATTO680, while little to no signals were detected at the tumor of MDA-MB-231-bearing mice up to 24 h post-injection. The TBR of Calu-3 tumors rapidly increased over time after systemic circulation of HER-ATTO680 (Figure 5b and Figure S11a). Interestingly, the background signals on skin in HER-ATTO680-treated Calu-3 bearing mice were similar to those in PBS-treated control mice (Figure S11). These results indicate that the quenched status of HER-ATTO680 was stably maintained during systemic circulation and the fluorescence of the conjugates turned on in the tumoral region, enabling early and clear discrimination of HER2-positive tumors from the surrounding normal tissues. The TBR of Calu-3 tumor was 4.8 and 8.1 at 5 h and 24 h post-injection, respectively, while only 1.8 ± 0.5 of TBR was observed for MDA-MB-231 tumors at 24 h post-injection. Figure 5c shows NIR fluorescence images of representative organs and tumors, supporting the specific accumulation of HER-ATTO680 in HER2-positive tumors and subsequent fluorescence activation in the tumor site.
Tumor-specific fluorescence imaging in xenograft tumor models. a) In vivo NIR fluorescence images of HER-ATTO680-treated mice. After intravenous injection of 50 µg/50 µL HER-ATTO680, NIR fluorescence images (λex 660/20 nm, λem 710/40 nm) of MDA-MB-231 and Calu-3 tumor-bearing mice were obtained. NIR fluorescence images of normal mice without tumors were also obtained after intravenous injection of PBS solution into the mice. b) Tumor-to-background ratios of fluorescence intensities (n = 3). c) Representative ex vivo fluorescence images of organs and tumors from Calu-3 and MDA-MB-231 tumor-bearing mice 1, 5, and 24 h after HER-ATTO680 administration (λex 660/20 nm, λem 710/40 nm).
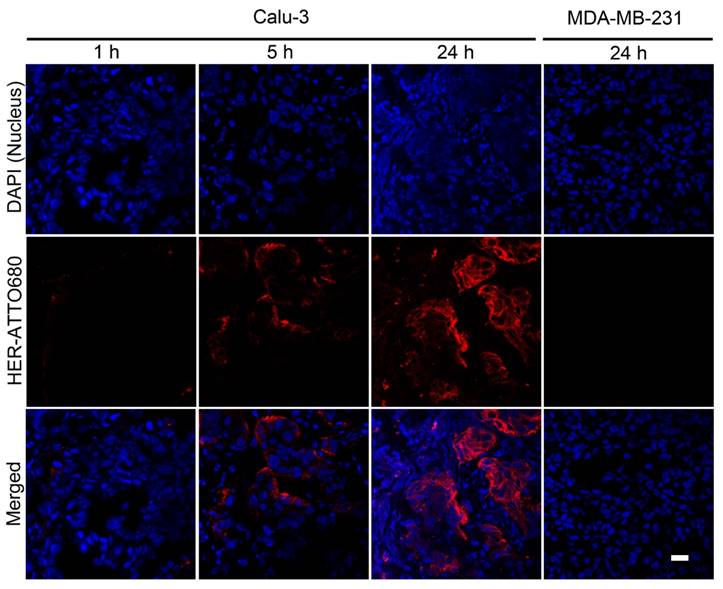
To find out the intratumoral microdistribution of targeted agents, finally, tumors were collected at each time point over the period of time after an intravenous injection of HER-ATTO680. Both Calu-3 and MDA-MB-231 tumors were resected and immersed into OCT compound to be sectioned at 7 µm thickness. After DAPI staining of nuclei, fluorescence images of tumorous tissues were acquired using a confocal scanning laser microscope (λex 633 nm and λem 647-754 nm for HER-ATTO680; λex = 405 nm and λem 420-480 nm for DAPI). As shown in Figure 6, a time-dependent increase in NIR fluorescence was observed in Calu-3 tumor sections, whereas no measureable fluorescence was observed in the MDA-MB-231 tissue. This result confirms that HER-ATTO680 accumulated in the tumor site after prolonged systemic circulation by the enhanced permeation and retention (EPR) effect, and thus real-time NIR fluorescence imaging was enabled.
As discussed above, the persistent background retention has been the major limitation of conventional antibody-fluorophore conjugates for their clinical applications. For example, IRDye800CW-conjugated bevacizumab and cetuximab have to be injected intravenously into patients 3 days prior to imaging because of the nonspecific uptake in major organs including liver, kidneys, and the gastrointestinal tract, which takes longer time to wash out completely from the background tissue [13,14]. Similarly, considerable background fluorescence was observed until 24 h post-injection in in vivo imaging experiment using HER-Alexa647 (Figure S12), which resulted in low TBR at 24 h time-point. In the practical point of view, performing image-guided surgery within 24 h post-injection of contrast agents is ideal not only because of reducing patient's hospitalization period, but also it can prevent potential photobleaching and biodegradation of the injected conjugates in the body. Our in vivo imaging study proves that target tumors could be discriminated within a day by using an antigen-responsive antibody conjugate: TBR of 4.8 and 8.1 at 5 h and 24 h post-injection of HER-ATTO680, respectively.
Confocal fluorescence microscopy images of tumor sections. Tumor tissues collected after 1, 5, and 24 h of HER-ATTO680 administration were prepared into 7-μm-thick sections. Nuclei in the tissue sections were counterstained with DAPI in mounting media. The DAPI (λex 405 nm, λem 420-480 nm) and HER-ATTO680 (λex 633 nm, λem 647-754 nm) images were pseudocolored in blue and red, respectively. Scale bar = 20 μm.
Conclusions
We successfully demonstrated the tumor-specific “off-on” fluorescence switch system of HER-ATTO680 bioconjugates in tumor cells and tumor-bearing mice. The NIR fluorescence of HER-ATTO680 was quenched at normal tissues via PET interactions between the fluorochrome and Trp or/and Tyr residues in the antibody. The bioconjugate signals turned on upon binding to the target antigen HER2 on the cancer cell surface, which enables fast and real-time fluorescence imaging of target cancer cells. The results from in vivo animal studies support the potential utility of HER-ATTO680 as an antigen-responsive targeted probe for fast and specific NIR fluorescence imaging of tumors and their image-guided therapy.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported by the National Cancer Center grant (1610150), and by the National Research Foundation of Korea (NRF) grant (NRF-2014R1A2A1A11050923) funded by the Korea government (MSIP), Republic of Korea.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics. 2000. CACancer J Clin. 2000;50:7-33
2. van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315-1319
3. Valdés PA, Roberts DW, Lu FK, Golby A. Optical technologies for intraoperative neurosurgical guidance. Neurosurg Focus. 2016;40:E8
4. Lim MC, Seo SS, Kang S, Kim SK, Kim SH, Yoo CW, Park SY. Intraoperative image-guided surgery for ovarian cancer. Quant Imaging Med Surg. 2012;2:114-117
5. Chang SJ, Bristow RE, Chi DS, Cliby WA. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol. 2015;26:336-342
6. Fass L. Imaging and cancer: a review. Mol Oncol. 2008;2:115-152
7. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJH. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507-518
8. Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW. et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323-332
9. Draney DR. Clinical trials in near infrared fluorescence imaging with IRDye 800CW. Proc SPIE. 2015;9311:93110L
10. Freise AC, Wu AM. In vivo imaging with antibodies and engineered fragments. Mol Immunol. 2015;67:142-152
11. Ogawa M, Regino CA, Choyke PL, Kobayashi H. In vivo target-specific activatable near-infrared optical labeling of humanized monoclonal antibodies. Mol Cancer Ther. 2009;8:232-239
12. Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res. 2011;44:83-90
13. Cohen R, Stammes MA, de Roos IH, Stigter-van Walsum M, Visser GW, van Dongen GA. Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets. EJNMMI Res. 2011;1:31
14. Korb ML, Hartman YE, Kovar J, Zinn KR, Bland KI, Rosenthal EL. Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. J Surg Res. 2014;188:119-128
15. Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F. et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31:148-153
16. Maass KF, Kulkarni C, Betts AM, Wittrup KD. Determination of cellular processing rates for a trastuzumab-maytansinoid antibody-drug conjugate (ADC) highlights key parameters for ADC design. AAPS J. 2016;18:635-646
17. Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, Marks JD, Adams GP. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res. 2011;71:2250-2259
18. Doose S, Neuweiler H, Sauer M. Fluorescence quenching by photoinduced electron transfer: a reporter for conformational dynamics of macromolecules. ChemPhysChem. 2009;10:1389-1398
19. Marme N, Knemeyer JP, Sauer M, Wolfrum J. Inter- and intramolecular fluorescence quenching of organic dyes by tryptophan. Bioconjugate Chem. 2003;14:1133-1139
20. Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K. et al. Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Br J Cancer. 1999;79:707-717
21. Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148-154
22. Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147-1157
23. Wong DJ, Hervitz SA. Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates. Ann Transl Med. 2014;2:122
Author contact
![]() Corresponding author: Yongdoo Choi, Ph.D. Tel: +82-31-920-2512 E-mail: ydchoire.kr.
Corresponding author: Yongdoo Choi, Ph.D. Tel: +82-31-920-2512 E-mail: ydchoire.kr.
 Global reach, higher impact
Global reach, higher impact