13.3
Impact Factor
Theranostics 2017; 7(4):814-825. doi:10.7150/thno.17366 This issue Cite
Research Paper
Theranostic Nucleic Acid Binding Nanoprobe Exerts Anti-inflammatory and Cytoprotective Effects in Ischemic Injury
1. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston MA;
2. Cardiovascular Research Center, Massachusetts General Hospital, Harvard Medical School, Boston MA.
3. Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston MA (H.C.: currently School of Materials Science and Engineering, Chonnam National University, Gwangju, South Korea).
4. Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston MA (currently Department of Anesthesiology, University of Maryland School of Medicine, Baltimore MD).
5. Division of Genetics and Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston MA.
Abstract
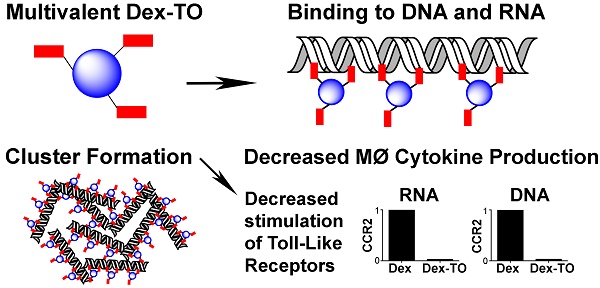
Extracellular nucleic acids are proinflammatory molecules that have been implicated in a diverse range of diseases. We report here the development of a multivalent nucleic acid scavenging nanoprobe, where the fluorochrome thiazole orange (TO) is conjugated to a polymeric 40 kDa dextran carrier. Dextran-TO (Dex-TO) has nanomolar affinity for mammalian and bacterial nucleic acids and attenuates the production of inflammatory cytokines from activated macrophages exposed to DNA and RNA. Mice with myocardial ischemia reperfusion that were treated with Dex-TO showed a decrease in myocardial macrophage infiltration at 24 hours (p<0.05) and a decrease in infarct size (18% ± 9%, p<0.01) on day 7. Dex-TO allows sites of injury to be identified with fluorescence imaging, while simultaneously exerting an anti-inflammatory and cytoprotective effect. Dex-TO could be of significant diagnostic and therapeutic (theranostic) utility in a broad range of conditions including ischemia, trauma, burns, sepsis and autoimmune disease.
Keywords: DNA, RNA, Inflammation, Ischemia, Nanoprobe, Imaging.
 Global reach, higher impact
Global reach, higher impact