13.3
Impact Factor
Theranostics 2017; 7(3):764-774. doi:10.7150/thno.15757 This issue Cite
Research Paper
Size-Tunable Gd2O3@Albumin Nanoparticles Conjugating Chlorin e6 for Magnetic Resonance Imaging-Guided Photo-Induced Therapy
1. Department of Radiology, Second Affiliated Hospital of Soochow University, Suzhou215004, Jiangsu, China;
2. Jiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases, and College of Pharmaceutical Sciences, Soochow University, Suzhou 215123, Jiangsu, China;
3. School for Radiological & Interdisciplinary Sciences (RAD-X), Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, and School of Radiation Medicine and Protection, Soochow University, Suzhou 215123, Jiangsu, China;
4. Department of General Surgery, Second Affiliated Hospital of Soochow University, Suzhou 215004, Jiangsu, China;
5. Institute of Radiotherapy & Oncology, Soochow University, Suzhou 215004, Jiangsu, China.
*Equal contribution to this study.
Abstract
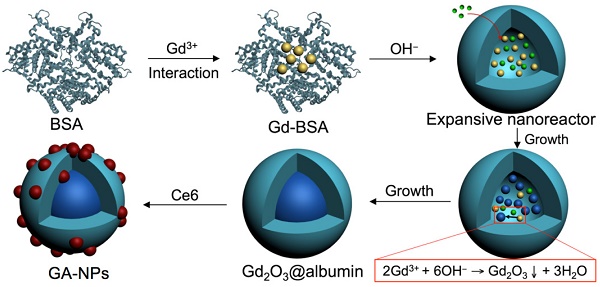
Protein nanoparticles as nanocarriers are of particular interest in the field of cancer therapy. Nevertheless, so far a facile fabrication of theranostic protein nanoparticles have been explored with limited success for cancer imaging and therapy. In this work, we demonstrate the controllable synthesis of size-tunable Gd2O3@albumin conjugating photosensitizer (PS) (GA-NPs) using hollow albumin as the nanoreactor for magnetic resonance imaging (MRI)-guided photo-induced therapy. The growth of Gd2O3 nanocrystals within the hollow nanoreactors is well regulated through reaction time, and a typical PS (e.g. chlorin e6) is further conjugated with the protein corona of the nanoreactor through facile chemical coupling, followed by the formation of theranostic GA-NPs. GA-NPs exhibit good longitudinal relaxivity, ideal photostability, enhanced cellular uptakes, and preferable size-dependent tumor accumulation. Moreover, GA-NPs effectively generate remarkable photothermal effect, intracellular reactive oxygen species from Ce6, and subsequent cytoplasmic drug translocation, thereby leading to severe synergistic photothermal and photodynamic cell damages. Consequently, GA-NPs exhibit an in vivo size-dependent MRI capacity with enhanced imaging contrast for effective tumor localization, and also generate a potent synergistic photodynamic therapy/photothermal therapy efficacy under irradiation owing to their enhanced tumor accumulation and strong photo-induced cytotoxicity. These results suggest that GA-NPs can act as a promising theranostic protein nanoplatform for cancer imaging and photo-induced therapy.
Keywords: albumin nanoreactor, gadolinium oxide, photosensitizer, magnetic resonance imaging, photodynamic therapy.
 Global reach, higher impact
Global reach, higher impact