13.3
Impact Factor
Theranostics 2016; 6(3):302-317. doi:10.7150/thno.13686 This issue Cite
Research Paper
Active Tumor Permeation and Uptake of Surface Charge-Switchable Theranostic Nanoparticles for Imaging-Guided Photothermal/Chemo Combinatorial Therapy
1. Department of Biomedical Engineering and Environmental Sciences, National Tsing Hua University, Hsinchu 30013, Taiwan
2. Department of Chemical Engineering, National Chung Hsing University, Taichung 402, Taiwan
Abstract
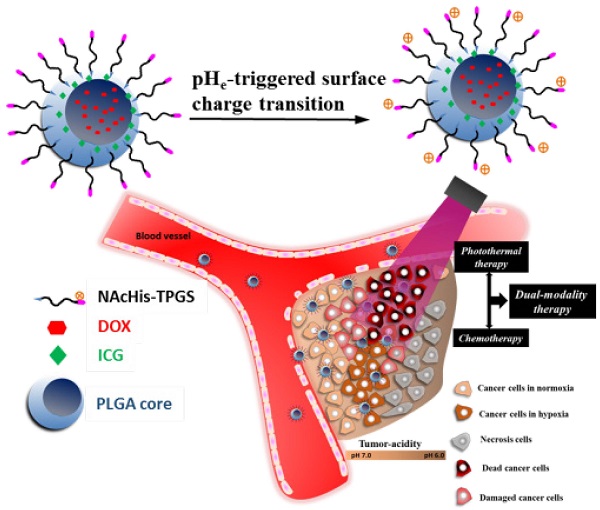
To significantly promote tumor uptake and penetration of therapeutics, a nanovehicle system comprising poly(lactic-co-glycolic acid) (PLGA) as the hydrophobic cores coated with pH-responsive N-acetyl histidine modified D-α-tocopheryl polyethylene glycol succinate (NAcHis-TPGS) is developed in this work. The nanocarriers with switchable surface charges in response to tumor extracellular acidity (pHe) were capable of selectively co-delivering indocyanine green (ICG), a photothermal agent, and doxorubicin (DOX), a chemotherapy drug, to tumor sites. The in vitro cellular uptake of ICG/DOX-loaded nanoparticles by cancer cells and macrophages was significantly promoted in weak acidic environments due to the increased protonation of the NAcHis moieties. The results of in vivo and ex vivo biodistribution studies demonstrated that upon intravenous injection the theranostic nanoparticles were substantially accumulated in TRAMP-C1 solid tumor of tumor-bearing mice. Immunohistochemical examination of tumor sections confirmed the active permeation of the nanoparticles into deep tumor hypoxia due to their small size, pHe-induced near neutral surface, and the additional hitchhiking transport via tumor-associated macrophages. The prominent imaging-guided photothermal therapy of ICG/DOX-loaded nanoparticles after tumor accumulation induced extensive tumor tissue/vessel ablation, which further promoted their extravasation and DOX tumor permeation, thus effectively suppressing tumor growth.
Keywords: surface charge transition, deep tumor penetration, tumor hypoxia, photothermal therapy, chemotherapy
 Global reach, higher impact
Global reach, higher impact