13.3
Impact Factor
Theranostics 2015; 5(8):834-846. doi:10.7150/thno.12040 This issue Cite
Research Paper
A Two-Step Pretargeted Nanotherapy for CD20 Crosslinking May Achieve Superior Anti-Lymphoma Efficacy to Rituximab
1. Department of Pharmaceutics and Pharmaceutical Chemistry, University of Utah, Salt Lake City, UT 84112, USA.
2. Institute for Stem Cell Biology and Regenerative Medicine, Stanford University, Stanford, CA 94305, USA.
3. Division of Hematology and Hematologic Malignancies and Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA.
4. Department of Bioengineering, University of Utah, Salt Lake City, UT 84112, USA.
† These authors contributed equally to this work.
Abstract
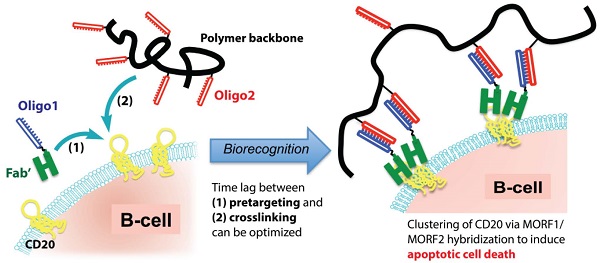
The use of rituximab, an anti-CD20 mAb, in combination with chemotherapy is the current standard for the treatment of B-cell lymphomas. However, because of a significant number of treatment failures, there is a demand for new, improved therapeutics. Here, we designed a nanomedicine that crosslinks CD20 and directly induces apoptosis of B-cells without the need for toxins or immune effector functions. The therapeutic system comprises a pretargeting component (anti-CD20 Fab' conjugated with an oligonucleotide1) and a crosslinking component (N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer grafted with multiple complementary oligonucleotide2). Consecutive treatment with the two components resulted in CD20 clustering on the cell surface and effectively killed malignant B-cells in vivo. To enhance therapeutic efficacy, a two-step pretargeting approach was employed. We showed that the time lag between the two doses can be optimized based on pharmacokinetics and biodistribution of the Fab'-oligonucleotide1 conjugate. In a mouse model of human non-Hodgkin lymphoma (NHL), increasing the time lag from 1 h to 5 h resulted in dramatically improved tumor growth inhibition and animal survival. When the 5 h interval was used, the nanotherapy was more efficacious than rituximab and led to complete eradication of lymphoma cells with no signs of metastasis or disease recurrence. We further evaluated the nanomedicine using patient mantle cell lymphoma cells; the treatment demonstrated more potent apoptosis-inducing activity than rituximab hyper-crosslinked with secondary antibodies. In summary, our approach may constitute a novel treatment for NHL and other B-cell malignancies with significant advantages over conventional chemo-immunotherapy.
Keywords: B-cell lymphoma, CD20, apoptosis, HPMA copolymer, rituximab, morpholino
 Global reach, higher impact
Global reach, higher impact