13.3
Impact Factor
Theranostics 2015; 5(7):667-685. doi:10.7150/thno.10438 This issue Cite
Research Paper
Characterization of Magnetic Viral Complexes for Targeted Delivery in Oncology
1. Department of Diagnostic and Interventional Radiology, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany;
2. Department of Experimental Oncology and Therapy Research, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany;
3. II. Med. Clinic, Gastroenterology, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany;
4. Research Unit Analytical Pathology, Institute of Pathology, Helmholtz Zentrum München, Neuherberg, Germany.
* contributed equally.
Abstract
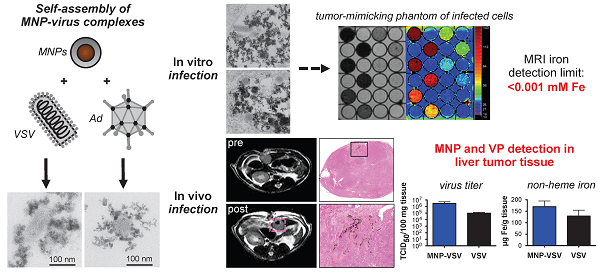
Oncolytic viruses are promising new agents in cancer therapy. Success of tumor lysis is often hampered by low intra-tumoral titers due to a strong anti-viral host immune response and insufficient tumor targeting. Previous work on the co-assembly of oncolytic virus particles (VPs) with magnetic nanoparticles (MNPs) was shown to provide shielding from inactivating immune response and improve targeting by external field gradients. In addition, MNPs are detected by magnet resonance imaging (MRI) enabling non-invasive therapy monitoring.
In this study two selected core-shell type iron oxide MNPs were assembled with adenovirus (Ad) or vesicular stomatitis virus (VSV). The selected MNPs were characterized by high r2 and r2* relaxivities and thus could be quantified non-invasively by 1.5 and 3.0 tesla MRI with a detection limit below 0.001 mM iron in tissue-mimicking phantoms. Assembly and cell internalization of MNP-VP complexes resulted in 81 - 97 % reduction of r2 and 35 - 82 % increase of r2* compared to free MNPs. The relaxivity changes could be attributed to the clusterization of particles and complexes shown by transmission electron microscopy (TEM). In a proof-of-principle study the non-invasive detection of MNP-VPs by MRI was shown in vivo in an orthotopic rat hepatocellular carcinoma model.
In conclusion, MNP assembly and compartmentalization have a major impact on relaxivities, therefore calibration measurements are required for the correct quantification in biodistribution studies. Furthermore, our study provides first evidence of the in vivo applicability of selected MNP-VPs in cancer therapy.
Keywords: magnetic viral complexes, nanoassembly, magnetic nanoparticles (MNPs), MRI relaxivity, MRI phantoms, oncolytic virus.
 Global reach, higher impact
Global reach, higher impact