13.3
Impact Factor
Theranostics 2015; 5(5):515-529. doi:10.7150/thno.10319 This issue Cite
Research Paper
Molecular Magnetic Resonance Imaging of Angiogenesis In Vivo using Polyvalent Cyclic RGD-Iron Oxide Microparticle Conjugates
1. Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, John Radcliffe Hospital, Oxford, OX3 9DU, United Kingdom.
2. Cancer Research UK and Medical Research Council Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, UK.
3. Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), Bethesda, Maryland 20892, United States.
*joint senior authors.
Abstract
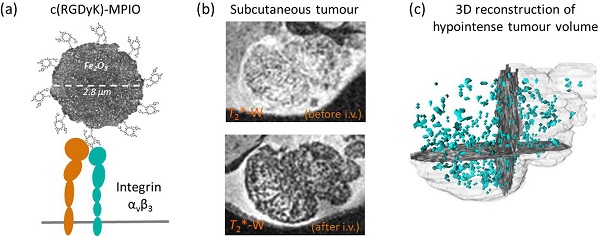
Angiogenesis is an essential component of tumour growth and, consequently, an important target both therapeutically and diagnostically. The cell adhesion molecule αvβ3 integrin is a specific marker of angiogenic vessels and the most prevalent vascular integrin that binds the amino acid sequence arginine-glycine-aspartic acid (RGD). Previous studies using RGD-targeted nanoparticles (20-50 nm diameter) of iron oxide (NPIO) for magnetic resonance imaging (MRI) of tumour angiogenesis, have identified a number of limitations, including non-specific extravasation, long blood half-life (reducing specific contrast) and low targeting valency. The aim of this study, therefore, was to determine whether conjugation of a cyclic RGD variant [c(RGDyK)], with enhanced affinity for αvβ3, to microparticles of iron oxide (MPIO) would provide a more sensitive contrast agent for imaging of angiogenic tumour vessels. Cyclic RGD [c(RGDyK)] and RAD [c(RADyK)] based peptides were coupled to 2.8 μm MPIO, and binding efficacy tested both in vitro and in vivo. Significantly greater specific binding of c(RGDyK)-MPIO to S-nitroso-n-acetylpenicillamine (SNAP)-stimulated human umbilical vein endothelial cells in vitro than PBS-treated cells was demonstrated under both static (14-fold increase; P < 0.001) and flow (44-fold increase; P < 0.001) conditions. Subsequently, mice bearing subcutaneous colorectal (MC38) or melanoma (B16F10) derived tumours underwent in vivo MRI pre- and post-intravenous administration of c(RGDyK)-MPIO or c(RADyK)-MPIO. A significantly greater volume of MPIO-induced hypointensities were found in c(RGDyK)-MPIO injected compared to c(RADyK)-MPIO injected mice, in both tumour models (P < 0.05). Similarly, administration of c(RGDyK)-MPIO induced a greater reduction in mean tumour T2* relaxation times than the control agent in both tumour models (melanoma P < 0.001; colorectal P < 0.0001). Correspondingly, MPIO density per tumour volume assessed immunohistochemically was significantly greater for c(RGDyK)-MPIO than c(RADyK)-MPIO injected animals, in both melanoma (P < 0.05) and colorectal (P < 0.0005) tumours. In both cases, binding of c(RGDyK)-MPIO co-localised with αvβ3 expression. Comparison of RGD-targeted and dynamic contrast enhanced (DCE) MRI assessment of tumour perfusion indicated sensitivity to different vascular features. This study demonstrates specific binding of c(RGDyK)-MPIO to αvβ3 expressing neo-vessels, with marked and quantifiable contrast and rapid clearance of unbound particles from the blood circulation compared to NPIO. Combination of this molecular MRI approach with conventional DCE MRI will enable integrated molecular, anatomical and perfusion tumour imaging.
Keywords: Tumour, angiogenesis, cRGDyK, microparticles, MRI, DCE.
 Global reach, higher impact
Global reach, higher impact