13.3
Impact Factor
Theranostics 2014; 4(1):81-89. doi:10.7150/thno.7193 This issue Cite
Review
Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target?
Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892, United States.
Received 2013-7-18; Accepted 2013-8-30; Published 2013-12-11
Abstract
Nano-sized therapeutic agents have several advantages over low molecular weight agents such as a larger loading capacity, the ability to protect the payload until delivery, more specific targeting due to multivalency and the opportunity for controlled/sustained release. However, the delivery of nano-sized agents into cancer tissue is problematic because it mostly relies on the enhanced permeability and retention (EPR) effect that depends on the leaky nature of the tumor vasculature and the prolonged circulation of nano-sized agents, allowing slow but uneven accumulation in the tumor bed. Delivery of nano-sized agents is dependent on several factors that influence the EPR effect; 1. Regional blood flow to the tumor, 2. Permeability of the tumor vasculature, 3. Structural barriers imposed by perivascular tumor cells and extracellular matrix, 4. Intratumoral pressure. In this review, these factors will be described and methods to enhance nano-agent delivery will be reviewed.
Keywords: Cancer, Nano-delivery, Tumor physiology, Enhanced permeability and retention effects.
1. Introduction
Nano-sized agents have a number of advantages over conventional low molecular weight agents including a large loading capacity, the ability to protect the payload from degradation, specific targeting and controlled or sustained release. (1-3) These features can be enhanced by changing characteristics such as size, the nature of the payload and surface features. (4,5) A variety of nano-sized, diagnostic and therapeutic agents have recently been synthesized for possible clinical application. (6-9) Nano-drugs are particularly relevant to cancer because tumors often possess a leaky vasculature compared with healthy vessels in normal organs. (10) When administered intravenously, nano-sized agents tend to circulate for longer times, if they are not small enough to be excreted by the kidney or large enough to be rapidly recognized and trapped by the reticuloendothelial system (RES). (11) Therefore, nano-sized agents with long circulation times leak preferentially into tumor tissue through a leaky tumor vasculature and are then retained in the tumor bed due to reduced lymphatic drainage. This process is known as the enhanced permeability and retention (EPR) effect. (12) Most nano-sized agents accumulate within tumors due to the EPR effect and then release their therapeutic payloads. However, EPR effects provide relatively modest specificity offering 20-30% increases in delivery compared with critical normal organs.
Nano-sized cancer drugs have shown efficacy in animal models of cancer and several agents are in testing in clinical trials. (13, 14) However, response rates vary, likely related to the broad heterogeneity of EPR effects observed among tumor types and within individual tumors. The aggregate EPR effect is dependent on factors, (15, 16) in which tumor-specific biological features are considered to affect the heterogeneity, including 1. The degree of angiogenesis and lymphangiogenesis. 2. The degree of perivascular tumor growth adjacent to the vasculature and the density of the stromal response. 3. Intratumoral pressure. By manipulating these conditions, EPR effects can be enhanced leading to superior nano-sized drug delivery, thereby enhancing their anti-cancer effects.
In this review, we first overview the basis of nano-sized drug delivery into cancer tissue, and then, discuss non-selective and selective molecular targeting methods for further improving the “permeability and retention” of nano-sized agents in cancer tissue compared with intrinsic EPR effects.
2. Physiology in tumor tissue
In normal tissues, low molecular weight agents enter a mature, organized hierarchical vascular network, beginning with arteries, continuing to arterioles and ending in capillaries, whereupon the agents leak from the vasculature and distribute homogenously within the tissue according to a concentration gradient. In solid tumors several factors inhibit the homogenous distribution of low molecular weight agents, particularly within deep and central parts of the tumor. In contrast to low molecular weight agents, nano-sized agents have a number of advantages in this setting.
In this section, we will describe the physiological characteristics of tumor tissues which present barriers to drug delivery, especially for non-targeted low molecular weight molecules (Figure 1).
2.1. Vascular structures
In order to grow, tumor cells recruit a neovasculature to ensure an adequate supply of nutrients and oxygen. As tumors grow they recruit new vessels or engulf existing blood vessels. The imbalance of pro- and anti-angiogenic signaling within different parts of tumors creates an abnormal vascular network that is characterized by dilated, tortuous, and saccular channels with haphazard patterns of interconnection and branching. (17-19) Unlike the microvasculature of normal tissue, which has an organized and regular branching order, tumor microvasculature shows disorganization and lack of the conventional hierarchy of blood vessels. (20) Arterioles, capillaries, and venules are not identifiable as such and instead, vessels are enlarged and are often interconnected by bidirectional shunts. (21) One physiological consequence of these vascular abnormalities is heterogeneity of tumor blood flow, (22) which interferes with the homogeneous distribution of a drug within the tumor.
Physiological characteristics of tumor tissue and vasculatures that can facilitate or prevent cancer drug delivery.
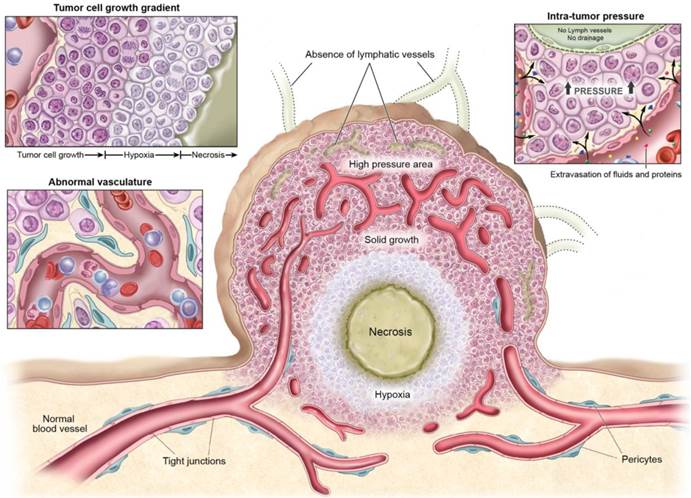
In addition to vascular heterogeneity, tumor blood vessels have structural abnormalities. The endothelial surface is fenestrated with gaps between endothelial cells, and is surrounded by discontinuous or absent basement membranes and fewer or poorly adherent pericytes. (23-25) Perivascular smooth muscle is often lacking in these vessels making them poorly reactive to stimuli. (26) The defective endothelial barrier function is one of the best-documented abnormalities of tumor vessels. (10) Blood vessel leakiness enables macromolecules to reach tumor cells from the bloodstream but also contributes to the high interstitial pressures in tumors which can inhibit accumulation of drugs in the tumor. (27)
2.2. Tumor cell growth
The heterogeneity of blood supply within the tumor microenvironment leads to marked gradients in the rate of cell proliferation; cancer cells near the vessels proliferate rapidly, while nutrient deprivation occurs in tumor cells located at or beyond the diffusion limit. This results in decreases in proliferation in cells distant from vessels while cellular density becomes higher near vessels. (28, 29) Microscopy reveals that tumor cells grow as sleeves or sheaths concentric with tumor vessels. (30) Such highly-cellular layers may interfere with drug penetration. (31) Treatments that induce apoptosis or necrosis within these perivascular tumor sheaths result in improved delivery of agents of all molecular sizes but gains are particularly noticeable with nano-sized agents. (32, 33)
2.3. Intra-tumoral pressure
Despite the barrier defect, central tumor vessels do not leak as much as expected due to the high interstitial pressure within tumors, which offsets the convective driving force favoring extravasation. High vascular permeability, coupled with mechanical compression of downstream blood vessels by tumor cell proliferation, causes an increase of interstitial fluids in the tumor. Furthermore, the lack of functional intratumoral lymphatic vessels inhibits the clearance of this extracellular fluid, further contributing to interstitial hypertension within tumors. (34) Elevated interstitial fluid pressure has been observed within various kinds of murine and human tumors. (35-37) In addition to inhibiting drug delivery by convection, increased interstitial fluid pressure further compresses blood vessels so that blood is diverted away from the center of the tumor toward the periphery.
3. Pharmacokinetics of nano-sized agents
The unique pharmacokinetics of nano-sized agents influences their delivery. Unlike small molecular drugs, nano-sized drugs slowly leak from blood vessels after intravenous injection. Therefore, in order to achieve satisfactory accumulation of drug in a tumor, sufficient time, typically measured in hours to days, must elapse before redosing. If the nano-sized agent is unstable in serum during this time, drug delivery will be inadequate. Persistent, unmodified, nano-drugs in the blood pool serve as a prolonged “input function” for the drug at the target. However, most nano-sized agents begin to be excreted or sequestered by the kidneys, liver, or reticuloendotherial system. Therefore, nano-sized agents should be appropriately designed to evade these clearance mechanisms enabling the agents to stay longer in the circulation. (4, 5)
Tissue perfusion is also critical for achieving successful therapy. Nano-sized drugs administered into the blood pool must leak efficiently out of vessels and homogeneously perfuse pathologic tissue. Regions with poor perfusion and leakiness may be underdosed.
In this section we will discuss the rational design of nano-sized agents that optimizes drug delivery, based on their physico-chemical properties (Figure 2).
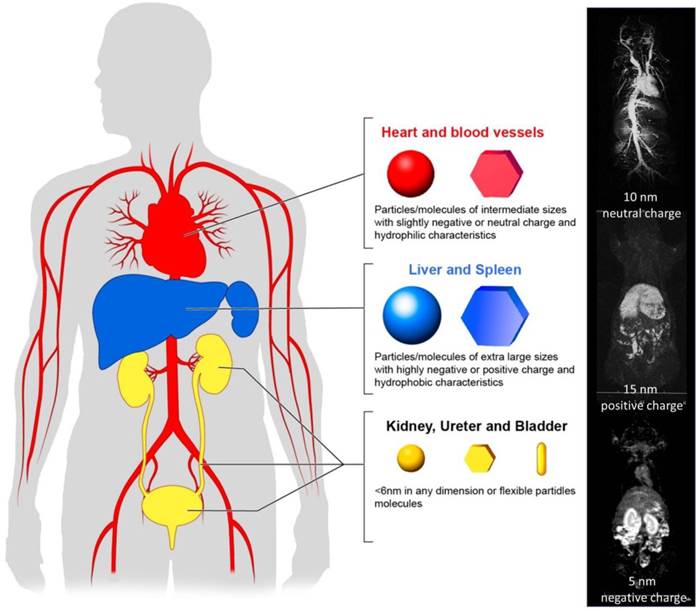
3.1. Kidney Excretion
The physiological function of the kidney is to filter the plasma at the glomerular basement membrane. Some molecules that are filtered at the glomerulus may be recovered in the proximal tubules and others that avoid filtration at the glomerulus may be excreted in the proximal tubules although this is rare for nano-sized agents. The kidney is highly efficient in filtering the plasma and therefore, glomerular filtration should be taken into account when designing nano-sized agents. The glomerular basement membrane is formed by specialized cells and connective tissues, and its surface is negatively charged. The glomerulus has mostly round pores of around 6 nm in diameter. (9, 11, 38) Therefore, the net charge of nano-sized agents close to 6 nm in size, will highly influence their renal excretion; positively charged or neutral molecules will be filtered more efficiently than negatively charged ones. (39) However, strongly positive-charged agents could be trapped by the brush border on proximal tubules than neutral or negatively charged agents. Additionally, the shape (40) and flexibility (hardness or softness) (41) of the agents will alter filtration, therefore, the hydrodynamic diameter measured by dynamic light scattering (DLS) alone may be insufficient to predict the degree of renal excretion. “Soft” molecules will more easily be filtered. Unfortunately, DLS measurements depict the average size, which may not accurately predict glomerular filtration since nano-sized agents typically have a range of sizes from below to above the 6 nm size threshold. In summary, nano-sized agents, which are more than 6 nm in shortest dimension, are predicted to be poorly excreted by the kidneys and therefore, should be retained in the circulation, provided they are not excreted rapidly in liver or phagocytosed by the RES. (5)
3.2. Liver and RES
The liver and RES recognize and remove foreign bodies from the blood pool. All nano-sized agents injected into the body are “foreign” substances. Therefore, nano-sized agents should be designed to be stealthy to evade rapid uptake by the liver or RES. Hydrophobic agents are frequently associated with serum proteins and are recognized and metabolized by the liver. (42) Larger molecules or particles are readily recognized by the RES. Molecules or particles with highly charged surfaces are also recognized by the RES and are quickly removed from the circulation. Therefore, useful design parameters of a nano-sized agent include limiting the size (probably to <300 nm in diameter), and maintaining a net charge as close to neutral as possible while providing a hydrophilic surface. (43) To achieve this design, hydrophilic and neutral polymers including polyethylene glycol and polysaccharides are commonly used on the surface of nano-sized agents to make them “stealthy” thereby evading from the recognition by the liver and RES. (44-46)
3.3. Leakage and tissue perfusion
Nano-sized particles preferentially leak into tumors compared with normal tissues due to the EPR effect. (47) However, tissue perfusion of nano-sized regents is less homogeneous in cancer tissue because of inhomogeneous vascular distribution/permeability in tumors. This leads to regions of hypoxia and necrosis which occur at a distance from the vessels. (48) Therefore, better leakage and deeper penetration can induce greater therapeutic effects.
Pharmacokinetics of nano-sized agents. Nano-sized agents, which are favorable for operating EPR effects, should stay in the blood pool for long time.
Methods for improving cancer nano-drug delivery based on EPR effects by manipulating intrinsic physiological barriers.
In general, extravascular leakage and tissue diffusion relies on thermodynamic movement of nano-sized molecules. Therefore, smaller molecules and particles perfuse deeper and more homogeneously into tumor tissue. However, in order to design nano-sized agents to meet the criteria described above, they must be made smaller, reducing their capacity to carry a therapeutic payload. Therefore, the optimal size of a nano-sized agent, which can deliver an adequate dose of drugs distributed homogeneously and thus, induce the most efficient therapeutic effect, is reportedly between 10 nm to 200 nm in diameter. (49)
4. Improving the enhanced permeability and retention (EPR) effect
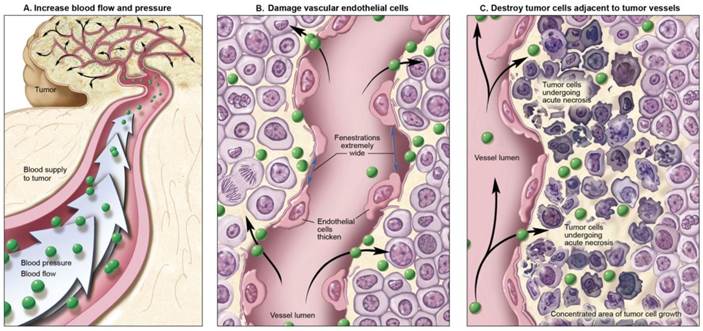
The previously described criteria for designing optimal nano-sized agents are based on the premise that a tumor arises de novo under physiologic conditions. When these natural conditions are artificially modified, unusually efficient drug delivery can be achieved. Three different conditions can be modified to improve nano-sized agent delivery; 1. Altering the normal physiologic condition without modifying the tumor environment. 2. Altering the condition of tumor vasculature or stroma. 3. Killing the cancer cells to reduce their barrier function (Figure 3). (50) Modification of one or more of these conditions can either improve or inhibit drug delivery, depending on local conditions.
4.1. Altering normal physiologic conditions
In order to improve the nano-drug delivery into cancer tissue and not normal tissue, one can increase the input function of the nano-sized agent. Normal vessels retain their ability to respond to extrinsic vasoconstrictors whereas tumor vessels lose their responsiveness to such agents. Muscular fibers in the vessel wall will contract, limiting blood flow in normal tissues. Therefore, when vasoconstrictive drugs are administered, normal vessels are constricted and blood pressure is increased. In contrast, tumor vessels do not respond to vasoconstrictors because of insufficient muscular structure. This leads to a relative increase in the input function into tumor tissues. (51-55) This phenomenon was recognized in 1970's during diagnostic angiography for tumor localization and was termed “pharmaco-angiography”. (56) During diagnostic angiography, vaso-constricting agents including alpha receptor agonists were injected to constrict normal vessels while accentuating tumor vessels. (57, 58) Later, pharmaco-angiography was used to constrict vessels after the delivery of nano-drug therapy to prolong the exposure of the tumor to the therapy. (59) Diagnostic pharmaco-angiography was supplanted by more sensitive techniques such as computed tomography and magnetic resonance imaging but the effect can still be put to use to selectively increase drug delivery.
4.2. Targeting tumor vasculature or stroma
Another approach for improving nano-drug delivery into cancer tissue is to physiologically modify the tumor vasculature. Several anti-angiogenic drugs have been approved and are in common use. Among them, the anti-vascular endothelial growth factor (VEGF) monoclonal antibody, bevacizumab, has been used for blocking the effect of VEGF thus, inhibiting tumor angiogenesis and suppressing tumor growth (60) by decreasing blood flow and vascular permeability. On the other hand, VEGF itself may temporally increase leakiness and perfusion in tumor tissue as a potential way to physiologically augment the EPR effect. (61) It has also been argued that anti-angiogenic treatment results in vascular normalization which improves the distribution of blood in the center of the tumor and thus, improves delivery of certain drugs. (62)
In recent work, researchers describe targeting the endothelial cells of the tumor vasculature by targeting the αvβ3 integrin using an RGD-peptide conjugated to a gold nano-particle. When light is applied, photo-thermal damage increases anti-tumor and EPR effects. (63) Similar effects have been seen with ultrasound microbubbles. (64) Damaging endothelial cells of the tumor vasculature might remove a barrier to drug delivery, however, it carries the risk of decreasing or even shutting down the tumor blood flow due to thrombosis thus, reducing the input function of drugs into tumors.
There are several other approaches to targeting the vasculature or stroma to promote vascular supply and vascular permeability in tumors, such as hyperthermia (65, 66), radiotherapy (67), high intensity focused ultrasound (68) and various mediators including bradykinin (52-55), nitric oxide-releasing agent (52-55, 69, 70), angiotensin-converting enzyme inhibitors (52-55), tumor necrosis factor α (69, 71), heme oxygenase-1 (53, 72) and proteases including collagenase (73) or hyaluronidase. (74) Most of these mediators are low molecular weight and thus, when injected systemically, will affect normal blood vessels in the vicinity of tumor, thus, facilitating extravasation not only within but also around tumors. A theoretical concern is that compromising the integrity of cancer stroma may promote metastasis. (50)
4.3. Killing tumor cells
Nano-drug delivery reportedly increases after many cancer therapies. The likely explanation for this is that tumor cells themselves act as a barrier to deeper penetration of nano-drugs. For instance, an one-time application of x-ray therapy, which damaged cancer cells but did not damage the vasculature in tumor tissue, increased the delivery of nano-sized molecules up to 2.2-fold at a peak of 8-12 hours after radiation. (75) That dose of radiation preferably killed well oxygenated cancer cells near vessels, therefore, temporarily increasing vascular permeability, when morphological changes were induced in perivascular cancer cells undergoing apoptosis. Interestingly, excess radiation damaged the vessels sufficiently to shut down blood flow and negatively affected nano-drug delivery. Similar vascular shut down has been reported during photo-dynamic therapy (PDT). (76) Since PDT damages both cancer cells and normal cells, PDT often reduces tumor blood flow. (77) Similar effects were observed with some chemotherapy including pacritaxel or docetaxel (78), which preferably killed tumor cells nearby blood vessels.
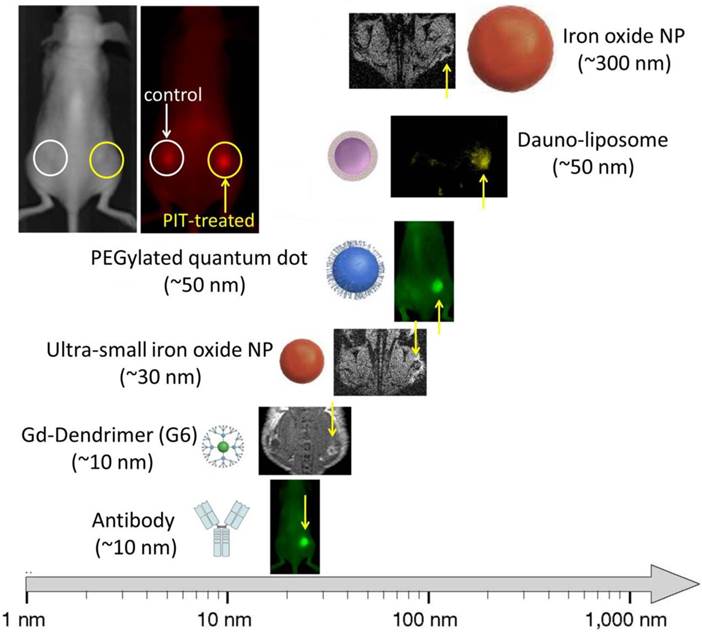
In recent work, selectively targeting tumor cells, researchers describe targeting the cancer cells via the GRP78 receptor using a GRP78-targeting peptide conjugated to a PEGylated gold nano-rod. When light was applied, photo-thermal damage increased EPR effects up to approximately 2-fold compared with untreated controls. (79) Furthermore, systemic radioimmunoconjugates preferably killed perivascular tumor cells resulting in improved drug delivery. (80, 81) However, these methods could also damage tumor vasculatures resulting in thrombotic occlusion from the bystander effect. More recently, another more selective method of killing tumor cells to augment drug delivery, named photo-immunotherapy (PIT), has been described (82). PIT can specifically kill cancer cells exposed to near infrared light by inducing immediate necrosis without damaging normal cells (including vascular endothelial cells). Since most of the initial cell killing occurs in the perivascular tumor sheaths, increases in nano-drug delivery up to 24-fold compared with untreated control tumors, can be observed. (83) This increased permeability was induced immediately after exposure to near infrared light. Dynamic fluorescence imaging showed that intravenously injected, non-targeted polyethylene glycol coated quantum dots (PEG-QD) quickly accumulated in the PIT-treated tumor bed compared with non-treated controls (Figure 4). Histology after PIT showed a markedly dilated tumor vasculature in the widened tumor interstitium along with cancer cell debris. Additionally, intravenously injected PEG-QD leaked throughout the cancer tissue. Thus, PIT induces immediate necrosis especially in the layers of cancer cells surrounding the tumor vasculature without damaging vascular cells themselves. This initially leads to decreased interstitial pressure and a commensurate rise in perfusion. Therefore, PIT induces selective damage to perivascular cancer tissues thus, markedly augmenting the EPR effect and dramatically increasing drug delivery. This super enhanced EPR has also been referred to as SUPR to distinguish it from conventional EPR.
5. Conclusion
Nano-sized cancer drugs are promising because they can be highly loaded with anti-cancer agents and intrinsically result in preferable tumor delivery based on the unmodified EPR effect. Several methods to improve nano-drug delivery into cancer tissue have been discovered. Those methods improved delivery by as much as approximately 2-fold compared with non-treated tumors. However, enhanced EPR effects which occur after PIT induces damage in the layers of cancer cells immediately adjacent to the tumor vasculature and have dramatic effects on perfusion with improvements in the delivery of nano-particles of up to 24-fold compared with untreated tumors. The magnitude of the nano-delivery improvement could have a direct impact on the therapeutic effects of nano-sized cancer drugs possibly resulting in dose reductions when used sequentially after PIT. Over all, more selective targeting of tumor vasculature that does not cause thrombosis would be a key for successful improvement of nano-drug delivery based on modified EPR effects.
Super EPR effect induced by photo-immunotherapy can deliver various nano-sized (10-200 nm) particles 15-24 fold concentration into tumor beds.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ng KK, Lovell JF, Zheng G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res. 2011;44:1105-13
2. Patel S, Bhirde AA, Rusling JF, Chen X, Gutkind JS, Patel V. Nano Delivers Big: Designing Molecular Missiles for Cancer Therapeutics. Pharmaceutics. 2011;3:34-52
3. Perche F, Torchilin VP. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J Drug Deliv. 2013;2013:705265
4. Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond). 2008;3:703-17
5. Longmire MR, Ogawa M, Choyke PL, Kobayashi H. Biologically optimized nanosized molecules and particles: more than just size. Bioconjug Chem. 2011;22:993-1000
6. Rose PG. Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarian cancer. Oncologist. 2005;10:205-14
7. McNerny DQ, Leroueil PR, Baker JR. Understanding specific and nonspecific toxicities: a requirement for the development of dendrimer-based pharmaceuticals. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:249-59
8. Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100:572-9
9. Kobayashi H, Brechbiel MW. Dendrimer-based macromolecular MRI contrast agents: characteristics and application. Mol Imaging. 2003;2:1-10
10. McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713-25
11. Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57:2271-86
12. Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21:797-802
13. Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M. et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007;104:3460-5
14. Riggio C, Pagni E, Raffa V, Cuschieri A. Nano-Oncology: Clinical Application for Cancer Therapy and Future Perspectives. J Nanomater. 2011;2011:164506
15. Lammers T, Kiessling F, Hwnnink WM, Storm G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175-87
16. Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653-64
17. Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947-70
18. Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206-25
19. Ziyad S, Iruela-Arispe ML. Molecular mechanisms of tumor angiogenesis. Genes Cancer. 2011;2:1085-96
20. Konerding MA, Miodonski AJ, Lametschwandtner A. Microvascular corrosion casting in the study of tumor vascularity: a review. Scanning Microsc. 1995;9:1233-43 discussion 43-4
21. Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685-93
22. Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641-58
23. Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP. et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752-6
24. Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P. et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35-52
25. Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985-1000
26. Chan RC, Babbs CF, Vetter RJ, Lamar CH. Abnormal response of tumor vasculature to vasoactive drugs. J Natl Cancer Inst. 1984;72:145-50
27. Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251-5
28. Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441-54
29. Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003;94:1021-8
30. Divan A, Lawry J, Dunsmore IR, Parsons MA, Royds JA. p53 and p21waf-1 expression correlates with apoptosis or cell survival in poorly differentiated, but not well-differentiated, retinoblastomas. Cancer Res. 2001;61:3157-63
31. Grantab R, Sivananthan S, Tannock IF. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res. 2006;66:1033-9
32. Au JL, Jang SH, Wientjes MG. Clinical aspects of drug delivery to tumors. J Control Release. 2002;78:81-95
33. Jang SH, Wientjes MG, Au JL. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: effect of treatment schedule. J Pharmacol Exp Ther. 2001;296:1035-42
34. Ji RC. Characteristics of lymphatic endothelial cells in physiological and pathological conditions. Histol Histopathol. 2005;20:155-75
35. Boucher Y, Salehi H, Witwer B, Harsh GRt, Jain RK. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br J Cancer. 1997;75:829-36
36. Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. 1992;52:6371-4
37. Roh HD, Boucher Y, Kalnicki S, Buchsbaum R, Bloomer WD, Jain RK. Interstitial hypertension in carcinoma of uterine cervix in patients: possible correlation with tumor oxygenation and radiation response. Cancer Res. 1991;51:6695-8
38. Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B. et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165-70
39. Kobayashi H, Le N, Kim IS, Kim MK, Pie JE, Drumm D. et al. The pharmacokinetic characteristics of glycolated humanized anti-Tac Fabs are determined by their isoelectric points. Cancer Res. 1999;59:422-30
40. Canelas DA, Herlihy KP, DeSimone JM. Top-down particle fabrication: control of size and shape for diagnostic imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:391-404
41. Ogawa M, Regino CA, Marcelino B, Williams M, Kosaka N, Bryant LH Jr. et al. New nanosized biocompatible MR contrast agents based on lysine-dendri-graft macromolecules. Bioconjug Chem. 2010;21:955-60
42. Muller RH, Wallis KH, Troster SD, Kreuter J. In vitro characterization of poly (methyl methacrylate) nanoparticles and correlation to their in vivo fate. J Control Release. 1992;20:237-46
43. Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71-9
44. Kataoka K, Kwon GS, Yokoyama M, Okano T, Sakurai Y. Block Copolymer Micelles as Vehicles for Drug Delivery. J Control Release. 1993;24:119-32
45. Hamidi M, Azadi A, Rafiei P. Pharmacokinetic consequences of pegylation. Drug Deliv. 2006;13:399-409
46. Akiyoshi K, Taniguchi T, Fukui H, Sunamoto J. Hydrogel Nanoparticle Formed by Self-Assembly of Hydrophobized Polysaccharide. Stabilization of Adriamycin by Complexation. Eur J Pharm Biopharm. 1996;42:286-90
47. Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189-207
48. Fenton BM, Paoni SF, Beauchamp BK, Ding I. Zonal image analysis of tumour vascular perfusion, hypoxia, and necrosis. Br J Cancer. 2002;86:1831-6
49. Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818-22
50. Marcucci F, Corti A. How to improve exposure of tumor cells to drugs — Promoter drugs increase tumor uptake and penetration of effector drugs. Adv Drug Deliver Rev. 2011;64:53-68
51. Suzuki M, Hori K, Abe I, Saito S, Sato H. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin II. J Natl Cancer Inst. 1981;67:663-9
52. Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliver Rev. 2011;63:136-51
53. Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J Control Release. 2012;164:138-44
54. Maeda H. Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:53-71
55. Maeda H. The link between infection and cancer: Tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci. 2013;104:779-89
56. Novak D, Weber J. [Pharmaco-angiography with angiotensin (author's transl)]. Rofo. 1976;124:301-9
57. Turini D, Nicita G, Fiorelli C, Selli C, Villari N. Selective transcatheter arterial embolization of renal carcinoma: an original technique. J Urol. 1976;116:419-21
58. Georgi M, Freitag B. [Pharmaco-angiography of liver tumours (author's transl)]. Rofo. 1980;132:287-93
59. Li CJ, Miyamoto Y, Kojima Y, Maeda H. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. Br J Cancer. 1993;67:975-80
60. Jordan BF, Runquist M, Raghunand N, Baker A, Williams R, Kirkpatrick L. et al. Dynamic contrast-enhanced and diffusion MRI show rapid and dramatic changes in tumor microenvironment in response to inhibition of HIF-1alpha using PX-478. Neoplasia. 2005;7:475-85
61. Cyran CC, Sennino B, Fu Y, Rogut V, Shames DM, Chaopathomkul B. et al. Permeability to macromolecular contrast media quantified by dynamic MRI correlates with tumor tissue assays of vascular endothelial growth factor (VEGF). Eur J Radiol. 2012;81:891-6
62. Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS. et al. Normalization of tumor blood vessels mproves the delivery of nanomedicines in a size-dependent manner. Nat Nanotech. 2012;7:383-8
63. Xie H, Diagaradjane P, Deorukhkar AA, Goins B, Bao A, Phillips WT. et al. Integrin alphavbeta3-targeted gold nanoshells augment tumor vasculature-specific imaging and therapy. Int J Nanomedicine. 2011;6:259-69
64. Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J Nucl Med. 2012;53:345-8
65. Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60:4440-5
66. Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancee Res. 2001;61:3027-32
67. Lammers T, Peschke P, Kuhnlei R, Subr V, Ulbrich K, Debus J. et al. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery systems. J Control Release. 2007;107:333-41
68. Ranjan A, Jacpbs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS. et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release. 2012;158:487-94
69. Seynhaeve ALB, Hoving S, Schipper D, Vermeulen CE, Aan de Wiel-Ambagtsheer G, Van Tiel ST. et al. Tumor necrosis factor α mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response. Cancer Res. 2007;67:9455-62
70. Seki T, Fang J, Maeda H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009;100:2426-30
71. Seki T, Carroll F, Illingworth S, Green Nicky, Cawood R, Bachtarzi H. et al. Tumour necrosis factor-alpha increases extravasation of virus particles into tumour tissue by activating the Rho A/Rho kinase pathway. J Control Release. 2001;156:381-9
72. Fang J, Qin H, Nakamura H, Ysukigawa K, Shin T, Maeda H. Carbon monoxide, generated by heme oxygenase-1, mediates the enhanced permeability and retention effect in solid tumors. Cancer Sci. 2012;103:535-41
73. Eikenes L, Bruland OS, Brekken C, De Lange Davis C. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human sarcoma xenografts. Cancer Res. 2004;64:4768-73
74. Eikenes L, Tari M, Tufto I, Bruland OS. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br J Cancer. 2005;93:81-8
75. Kobayashi H, Reijnders K, English S, Yordanov AT, Milenic DE, Sowers AL. et al. Application of a macromolecular contrast agent for detection of alterations of tumor vessel permeability induced by radiation. Clin Cancer Res. 2004;10:7712-20
76. Wang KK, Cottrell WJ, Mitra S, Oseroff AR, Foster TH. Simulations of measured photobleaching kinetics in human basal cell carcinomas suggest blood flow reductions during ALA-PDT. Lasers Surg Med. 2009;41:686-96
77. Dubreta K, Ivankovic S, Lovrencic-Huzjan A, Bosnar-Puretic M, Stojkovic R, Jurin M. The characterization of blood flow changes in mouse tumor during Photofrin-based photodynamic therapy by using the color Doppler ultrasonography. Oncol Rep. 2009;22:1253-7
78. Taghian AG, Abi-Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E. et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J Clin Oncol. 2005;23:1951-61
79. Gormley AJ, Larson N, Sadekar S, Robinson R, Ray A, Ghandehari H. Guided Delivery of Polymer Therapeutics Using Plasmonic Photothermal Therapy. Nano Today. 2012;7:158-67
80. Clarke K, Lee F-T, Brechbiel MW, Smyth FE, Old LJ, Scott AM. Therapeutic efficacy of anti-Lewisy humanized 3S193 radioimmunotherapy in a breast cancer model: enhanced activity when combined with taxol chemotherapy. Clin Cancer Res. 2000;6:3621-8
81. DeNardo SJ, Kukis DL, Kroger LA, O'Donnell RT, Lamborn KR, Miers LA. et al. Synergy of Taxol and radioimmunotherapy with yttrium-90-labeled chimeric L6 antibody: Efficacy and toxicity in breast cancer xenografts. Proc Natl Acad Sci USA. 1997;94:4000-4
82. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685-91
83. Sano K, Nakajima T, Choyke PL, Kobayashi H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano. 2013;7:717-24
Author biography
Hisataka Kobayashi is the Chief of the Molecular Theranostic Laboratory, in the Molecular Imaging Program at the National Cancer Institute of the National Institutes of Health in Bethesda, MD. Dr. Kobayashi received both an MD and PhD (Immunology/Medicine) from the Kyoto University in Japan. After a postodoctoral fellowship in the Nuclear Medicine Department at the Clinical Center of the National Institutes of Health he moved to his current position in the Molecular Imaging Program at NCI in 2004. His interest is in developing novel molecular imaging and therapy (theranostic) agents and technologies especially for targeting cancers.
Rira Watanabe is a research scholar in the Molecular Imaging Program at the National Cancer Institute of the National Institutes of Health in Bethesda, MD. Dr. Watanabe graduated from the Hokkaido University in Japan and earned a PhD in veterinary medicine. She works in a pharmaceutical company as a senior researcher and is currently on sabbatical in the Molecular Imaging Program as a special volunteer. Her interest is in developing therapeutic methods through novel imaging techniques in oncology.
Peter L. Choyke is the Director of the Molecular Imaging Program at the Center for Cancer Research of the National Cancer Institute in Bethesda, MD. Dr. Choyke is a graduate of Jefferson Medical School and received training in Diagnostic Radiology at Yale University and the University of Pennsylvania. He joined the Diagnostic Radiology Department at the Clinical Center of the National Institutes of Health in 1988 and formed the Molecular Imaging Program at NCI in 2004. His interest is in accelerating the treatment of cancer by using novel molecular imaging agents which target specific features of cancers.
![]() Corresponding author: Hisataka Kobayashi, M.D., Ph.D. Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, NIH, Building 10, RoomB3B69, MSC1088, Bethesda, MD 20892-1088. Phone: 301-451-4220, Fax: 301-402-3191. E-mail: Kobayashnih.gov.
Corresponding author: Hisataka Kobayashi, M.D., Ph.D. Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, NIH, Building 10, RoomB3B69, MSC1088, Bethesda, MD 20892-1088. Phone: 301-451-4220, Fax: 301-402-3191. E-mail: Kobayashnih.gov.
 Global reach, higher impact
Global reach, higher impact