13.3
Impact Factor
Theranostics 2012; 2(2):179-189. doi:10.7150/thno.3716 This issue Cite
Research Paper
Optical Imaging of Cancer-Related Proteases Using Near-Infrared Fluorescence Matrix Metalloproteinase-Sensitive and Cathepsin B-Sensitive Probes
1. Center for Theragnosis, Biomedical Research Institute, Korea Institute of Science and Technology (KIST), Seoul 136-791, Korea
2. Center for Bionics, Biomedical Research Institute, Korea Institute of Science and Technology (KIST), Seoul 136-791, Korea.
* These authors contributed equally to this paper.
Received 2011-10-27; Accepted 2011-12-2; Published 2012-2-10
Abstract
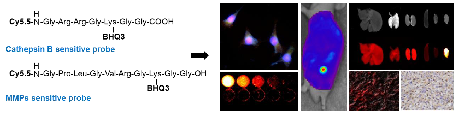
Cathepsin B and matrix metalloproteinase (MMP) play key roles in tumor progression by controlled degradation of extracellular matrix. Consequently, these proteases have been attracted in cancer research, and many imaging probes utilizing these proteases have been developed. Our groups developed cathepsin B and MMP imaging nanoprobes based on polymer nanoparticle platform. Both cathepsin B and MMP imaging probes used near-infrared fluorescence (NIRF) dye and dark-quencher to for high sensitivity, and protease-sensitive peptide sequence in each probe authorized high specificity of the probes. We compared the bioactivities of cathepsin B and MMP sensitive probes in cancer-related environments to investigate the biological property of the probes. As a result, cathepsin B probe showed fluorescence recovery after the probe entered the cytoplasm. This property could be useful to evaluate the cytoplasmic targeted delivery by using probe-conjugated nanoparticles in vivo. On the other hand, MMP probe was superior in specificity in vivo and tissue study. This comparative study will provide precise information about peptide-based optical probes, and allow their proper application to cancer diagnosis.
Keywords: Cathepsin B, matrix metalloproteinase, polymer nanoparticle
Introduction
Recently molecular imaging has been greatly progressed for biomedical application, and the expression or activity of specific enzymes including protease can be monitored by molecular imaging techniques [1-3]. Especially, near-infrared fluorescence (NIRF) imaging with small animals has been widely studied in the last decade due to its relatively deep tissue penetration. Various NIRF imaging probes were designed and applied for diagnosis of diseases, and some of them are enzyme-sensitive probes which can change their signal intensity in response to specific enzymes in targeted site [4-9]. These probes can monitor the enzymatic activities, and they also can be used as an efficient optical contrast agent for enzyme rich target site. Importantly, many enzymes, such as caspases, secretases, furin, phosphatases, and other proteases have been major targeted for optical probe [10-13], because they are expressed in tissues with pathological changes. They also have various biological mechanisms and catalytic activities related with progression of disease like inflammation, immune disorder, and tumors. Especially, in cancer imaging, monitoring the activity of specific protease is closely relevant to tumor progression.
Several proteases, including cathepsin B, urokinase-type plasminogen activator, and matrix metalloproteinases (MMPs) are involved in tumor progression [14, 15]. Among them, MMPs are family of metalloproteinases which need zinc ion essentially on their active-sites. Most MMPs are initially secreted as inactivated forms (proMMPs), and proMMPs are converted functionally active forms in extracellular space. Collagenase-1 (MMP-1), collagenase-2 (MMP-8), and collagenase-3 (MMP-13) are typical MMPs, and they actively participate in tissue remodeling for tumor progression [16, 17]. Moreover, MMPs also play an essential role in survival of cancer cells [18], and they are overexpressed in various cancer including colon, pancreas, and breast cancers [19-21]. On the other hand, cathepsin B is another protease that plays key roles in growth, migration, invasion, and metastasis of cancer cells [22, 23]. This lysosomal cysteine protease is overexpressed in cancer cells, such as gastric or colon cancers [24, 25]. Squamous cell carcinoma cells (SCC-7) was also confirmed that it express high levels of cathepsin B [26, 27]. Because overexpressed MMPs and cathepsin B play important biological functions in tumor, many imaging probes targeting these proteases have been developed for cancer diagnosis [28, 29].
We developed two different protease-sensitive optical probes which showed enhanced fluorescence signal intensity by cathepsin B and MMPs, respectively [6, 7, 30]. When these probes were exposed to the specific proteases at the tumor region, they induced sensitive and specific fluorescence recovery in tumor. Our two probes adopted similar structural design with NIR fluorochrome and dark quencher. Both probes utilized quenching/dequenching system with fluorochrome dye and dark quencher to provide superior sensitivity, but the dequenching conditions of two probes could be distinguished from each other for biological properties of each target protease. The understanding of the precise dequenching conditions of each probe is important to make an accurate cancer diagnosis. Furthermore, appropriate application of each protease-sensitive optical probe could provide more valuable information in cancer research.
Most studies had been focused on the chemical properties of single probe, and only a few comparative studies related with the biological property of the probes had been performed. However, understanding the biological properties and differences in dequenching condition of probes are important to use the probes appropriately. Herein, we investigated and described the behaviors of two specific protease-sensitive probes under the various conditions including cell culture supernatant, in vitro, in vivo, and ex vivo. We compared two parallel designed probes with different target protease under the identical conditions, and this comparative study on the two probes is expected to provide advantages of each probe and to guide their proper application in cancer diagnosis.
Materials and methods
Preparation of cathepsin B and MMP probes
Cathepsin B-sensitive NIRF probe (cathepsin B probe) and MMPs-sensitive NIRF probe (MMP probe) were prepared as previously described [6, 7]. Briefly, each probe was synthesized by conjugating NIR fluorochrome (Cy5.5) and dark quencher (black hole quencher-3, BHQ-3) to a cathepsin B-sensitive (Gly-Arg-Arg-Gly-Lys-Gly-Gly) or a MMPs-sensitive (Gly-Pro-Leu-Gly-Met-Arg-Gly-Leu-Gly-Lys) substrate protein. The substrate peptides were synthesized by standard solid-phase Fmoc peptide chemistry (Peptron, Daejeon, Korea). The final probe was characterized by analytical reverse phase-high performance liquid chromatography (RP-HPLC; Purity: >95%), UV-Vis spectra, and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) [7].
To confirm the correlation of the probe activities with the protease concentration, each probe (20 nM) was incubated with various concentrations of each protease in the sterile 96-well microplate (Greiner Bio-One, Tokyo, Japan). After incubation of the microplate at 37 ºC for 30 minutes, the fluorescence intensity was measured with a Kodak Imaging Station 4000 MM (New Haven, CT).
Immunocytochemistry and NIRF signal recovery of probes in cultured cells
To confirm the colocalization of each protease expression and probe signal in cellular level, immunocytochemistry using MMP-13 or cathepsin B antibody was performed after probe treatment. The MMP probe was confirmed to be dequenched by various types of MMP including MMP-2 and MMP-13, and we selected MMP-13 antibody because the MMP probe was more sensitive to MMP-13 than other MMPs [6].
Murine squamous cell carcinoma cells (SCC-7) were obtained from the American Type Culture Collection (Rockville, MD) and used in this study. The cells were cultured in RPMI 1640 media (Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY) and 1% penicillin/streptomycin at 37 °C in a humidified 5% CO2 atmosphere. SCC-7 cells were seeded at a density of 5×104 cells/dish in 35 mm cover-glass bottom dishes and incubated for 36 hours. After incubation, cathepsin B probe (200 nM) or MMP probe (200 nM) was added into each culture dish and incubated for 3 hours. Subsequently, the cells were rinsed with phosphate buffered saline (pH 7.4, PBS) and fixed in 3.5 % paraformaldehyde in PBS for 10 minutes at room temperature (RT). The antigens were retrieved in 95°C antigen retrieval buffer (100mM Tris, 5% urea, pH 0.5) for 10 minutes. After 3-times of washes, the cells were permeablized in 0.25% Triton X-100 in PBS for 5 minutes at RT. The permeabilized cells were incubated with 1% bovine serum albumin in PBS for 30 minutes to block unspecific binding of the antibodies and each dish was incubated in the 1:200 diluted anti-cathepsin B mouse IgG (Abcam, Cambridge, MA) or anti-MMP-13 mouse IgG (Calbiochem, Nottingham, UK) for 2 hours. We used the cells which omitted each primary antibody as a negative control. The anti-mouse IgG-FITC (1:1,500 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA) was applied to each dish for 40 minutes in dark condition. After 3 times of wash in PBS, the nuclei of the cells were stained with DAPI. The fluorescence images were observed with IX81-ZDC focus drift compensating microscope with FITC and Cy5.5 filters at 600× magnification (Olympus, Tokyo, Japan).
NIRF recovery of the probes in cell culture supernatant
To compare the activities of two probes in extracelluar space, cell supernatant was collected and reacted with various concentrations of each probe. First-passaged SCC-7 cells were cultured until confluence, and then switched to serum-free medium for 20 hours before the harvest of cell culture supernatant. Then, 90 μl aliquot of supernatant was reacted with various concentrations (from 3.125 nM to 50 nM) of cathepsin B or MMP probe in the sterile 96- well microplate (Greiner Bio-One, Tokyo, Japan). As a control experiment, the same amount (90 μl) of distilled water was also reacted with identical concentrations of each probe and compared with the result of reacted culture supernatant. The 96-well microplate was incubated at 37 ºC for 30 minutes, and the fluorescence intensity was monitored using a Kodak Imaging Station 4000 MM (New Haven, CT) equipped with filter set for Cy 5.5 after incubation. The excitation wave length was set at 675 nm and emission spectra recorded from 680 to 720 nm. The signal intensity was acquired from the fluorescence image sections of a microplate. The NIRF intensity was expressed as a mean intensity of selected area.
In vivo NIRF Imaging of the probes injected tumor bearing mice
To evaluate the dequenching of probes in vivo, SCC-7 tumor-bearing nude mice (5 weeks old, n=5 per each group, Institute of Medical Science, Tokyo) were prepared. SCC-7 cells (1×106 cells/mouse) suspended in 50 μl sterile media were injected subcutaneously into the left flank of mice. When the diameter of tumors reached approximately 7.0 ± 0.5 mm in size, 100 μl of cathepsin B probe (1 mg/ml of saline) or MMP probe (1 mg/ml of saline) was injected intravenously via a tail vein. After intravenous injection, the NIRF signals were non-invasively monitored using the eXplore Optix imaging system (ART Advanced Research Technologies Inc., Montreal, Canada) as previously described [31]. Imaging was performed 1, 3, 6, 9, and 12 hours post injection, and the acquired images were assessed with an Analysis Workstation (ART Advanced Research Technologies Inc.). For the comparison of fluorescence intensity at tumor of cathepsin B or MMP probe injected mice, paired t-test was used. All animal care and experimental procedures were performed in compliance with the Institutional guidelines of Korea Institute of Science and Technology (KIST) and the relevant laws, and institutional committees have approved the experiments.
Immunohistochemistry and NIRF signal recovery of probes in organ and tissues
To further assess the NIRF signal intensity in the body, one mouse of each group was euthanized at the time point that the NIRF signal at the tumor showed peak intensity. Then, tumors and other visceral organs from each mouse were excised to acquire ex vivo NIRF images with KODAK image station. After in vivo and ex vivo imaging, tumor and major internal organs are divided into 2 pieces. The one was fixed in neutral buffered formalin and embedded in paraffin, and the other piece was embedded in optimum cutting temperature tissue compound (OCT compound, Sakura, Tokyo) for cryosection preparation.
Immunohistochemistry (IHC) using cathepsin B and MMP-13 antibodies is performed to confirm the accordance of NIRF signal recovery of probe and cathepsin B or MMP expression in tissues. Routine IHC was performed using 6 μm-thick sections of formalin fixed and paraffin embedded tissues. The slides were deparaffinized and rehydrated, then endogenous peroxidase activities were blocked with 3% H2O2 solution for 20 minutes. The primary antibodies were applied for 2 hours RT, and HISTOSTAIN®-PLUS Kit (Invitrogen, Carlsbad, CA) was applied following the manufacture's instruction to detect protein expressions. After the processes, each slide was counter-stained with Harris hematoxylin.
For the evaluation of NIRF signal recovery of probes in tissues, the 8 μm-thick frozen sections were prepared for fluorescence microscopic imaging of probe dequenching in tissues including tumor. The slides were observed under the IX81-ZDC focus drift compensating microscope with Cy 5.5 filter set without any histological stains.
Result
Preparation of cathepsin B and MMP probes
The NIRF imaging mechanism and structure of cathepsin B probe and MMP probe are shown in Figure 1A and B. On the basis of substrate peptide of MMPs and cathepsin B, the probes was prepared by conjugation of linker peptides, NIRF dye (Cy5.5), and dark quencher (BHQ-3) [6, 7]. The cleavage of linker peptides by specific protease induces an activation of probe to recover fluorescence at target site (Figure 1A). When the inactive form of probes reaches to target tissues, the activity of target protease can be imaged through the cleavage of peptide and recovery of their fluorescence. These activatable probes showed high specificity and sensitivity by reducing background fluorescence.
We observed the fluorescence change of each probe with addition of the target protease (2 to 8 nM). After 30 minutes incubation, 4 nM of cathepsin B probe showed 8.9-times higher fluorescence intensity than the probe incubated with cathepsin B inhibitor (Figure 1C). The MMP probe with 4 nM of MMP-13 also showed 8.8-times higher fluorescence intensity than the probe incubated with MMP-13 inhibitior (Figure 1D). The fluorescence intensity of each probe was increased according to the increase of the corresponding protease. The cathepsin B probe which was incubated with 2, 4, and 8 nM of cathepsin B showed 13.5, 31.2, and 50.0 times higher fluorescence intensity than the probe incubated without cathepsin B. MMP probe with 2, 4, and 8 nM of MMP-13 also showed 13.4, 24.7, and 48.4 times higher fluorescence intensity than the probe incubated without MMP-13.
(A) NIRF recovery of the cathepsin B and MMP probes by target enzyme. (B) Structural designs of cathepsin B probe and MMP-2 probe. (C) The NIRF recovery of cathepsin B probe by 2nM, 4nM, and 8 nM of cathepsin B in vitro. (D) The NIRF recovery of MMP probe by 2nM, 4nM, and 8 nM of MMP-13 in vitro.
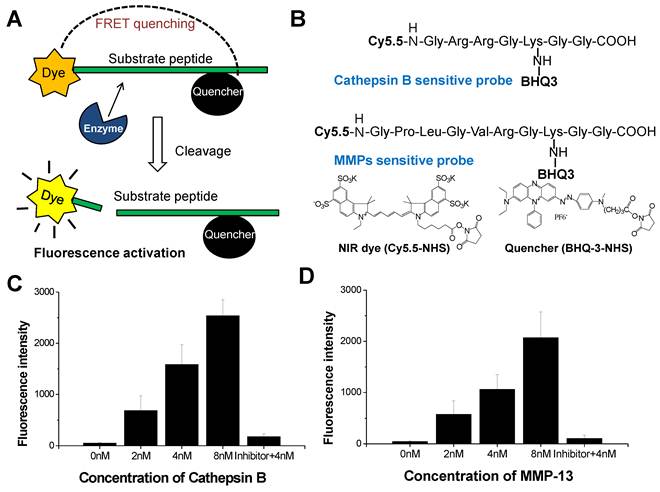
The cathepsin B expression and the NIRF signal of cathepsin B probe in cultured SCC-7 cells. (A) DIC image of the SCC-7 cells. (B) Nuclei of the SCC-7 cells which were stained with DAPI. (C) Cathepsin B expression in the cytoplasm of the cultured SCC-7 cells. (D) NIRF signal of the cathepsin B probe in the SCC-7 cells. (E) The coincidence of cathepsin B expression and the probe signal in merged image.
Cathepsin B/MMP-13 expression and NIRF signal recovery of probes in SCC-7 cells
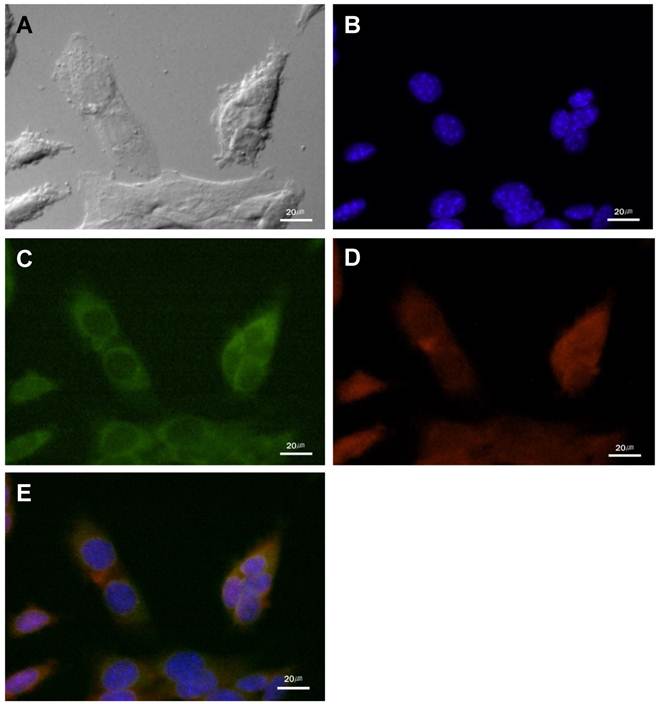
In cultured SCC-7 tumor cells, NIRF signal recovery of two probes was closely related with expression of corresponding protease. Immunocytochemistry demonstrated the location of cathepsin B and MMP-13 in probe-treated SCC-7 tumor cells. Cathepsin B expression was observed in the cytoplasm of the tumor cells (Figure 2). The recovered NIRF signal was also limited in the cytoplasm and completely coincided with cathepsin B expression (Figure 2E). On the other hand, intense MMP-13 expression was observed in the nuclei of the cells than cytoplasm, which is agreed with the NIRF signal recovery of MMP probe (Figure 3). No significant cathepsin B/MMP-13 positive signals were observed in negative control.
NIRF signal recovery of Cathepsin B and MMP probes in cell culture supernatant
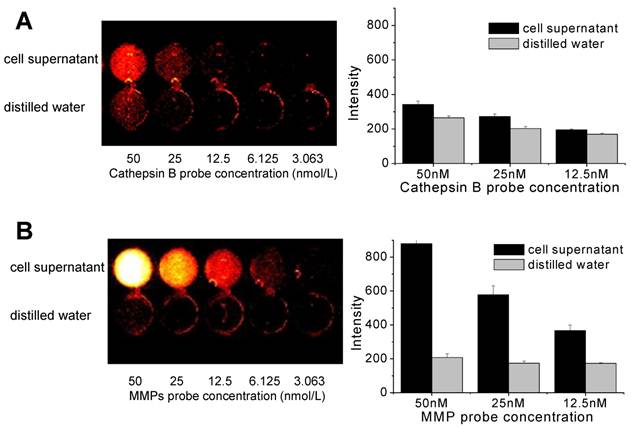
When the various concentrations of cathepsin B probes were added to cell culture supernatant, the NIRF signals was not recovered distinctly (Figure 4A). The fluorescent signal intensity of cathepsin B probe in cell culture supernatant was similar with that in distilled water at 50, 25, and 12.5 nM of probe concentration. However, MMP probe displayed distinct NIRF recovery in cell culture supernatant (Figure 4B). At 50 nM of MMP probe concentration, the cell supernatant showed more than 4-fold higher NIRF intensity compared to distilled water. The degree of increased NIRF recovery was dependent upon the concentration of added probe. These data showed that dequenching condition of two probes in cell culture supernatant was definite, which is may be originated from the secretion of MMP from cells.
The MMP-13 expression and NIRF signal of the MMP probe in cultured SCC-7 cells. (A) DIC image of the SCC-7 cells. (B) Nuclei of the SCC-7 cells which were stained with DAPI. (C) MMP-13 expression in the cultured SCC-7 cells. (D) NIRF signal of the MMP probe in the SCC-7 cells. (E) The co-localization of MMP-13 expression and MMP probe signal in merged image.
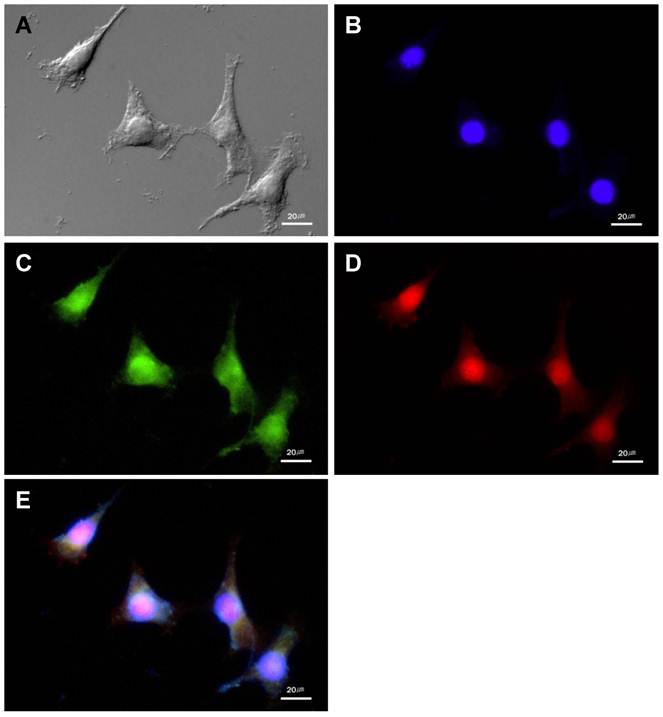
NIRF recovery of cathepsin B and MMP probes in cell culture supernatant. (A) Quenching properties and low NIRF recovery of cathepsin B probe in culture supernatant at various concentrations of the probe. (B) High NIRF recovery of MMP probe in culture supernatant at various concentrations of the probe. fluorescence imaging of 96-well microplate (695 nm).
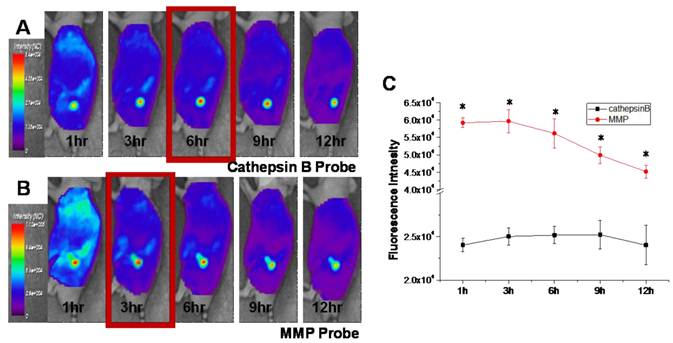
In vivo NIRF Imaging of cathepsin B/MMP probe injected tumor bearing mice
Figure 5 shows serial images of mice obtained for 12 h after intravenous injection of the cathepsin B and MMP probes. Cathepsin B probe displayed higher fluorescent intensity in tumor site for 12 hours. The NIRF at the tumor site of cathepsin B probe injected mice was gradually increased up to 6 hours after probe injection (Figure 5A). The MMP probe also showed high and specific NIRF signal recovery in tumor site of the probe injected tumor-bearing mice. Meanwhile, the maximum NIRF intensity in tumor site of the MMP probe-injected mice was observed at 3 hours post injection. The peak fluorescence intensity of the cathepsin B and MMP probes injected mice was significantly different (p<0.05), and the gap of the time point showing maximum NIRF intensity was that MMP probes were activated 3 hours earlier than cathepsin B probes in vivo.
Cathepsin B/MMP-13 expressions and NIRF signal recovery of probes in tumor and other organs
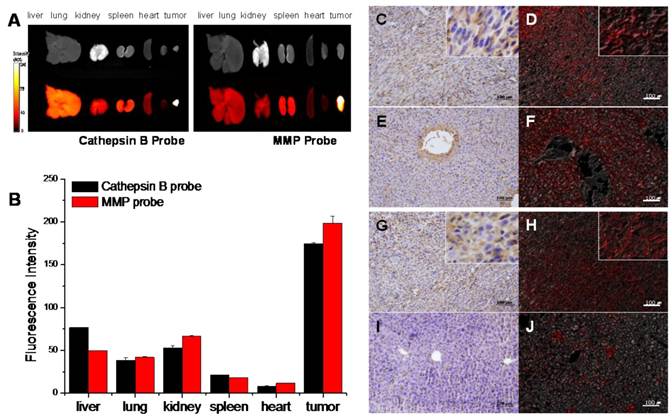
The ex vivo images were closely correlated with the in vivo images, and they reconfirmed that both probes were dequenched in tumor site with high specificity. However, notably, the cathepsin B probe displayed relatively higher NIRF signals in visceral organs including liver and kidney. On the contrary, MMP probe had relatively lower signals in the visceral organs compared to cathepsin B probe (Figure 6A and B).
Cathepsin B and MMP-13 expressions in the excised organs including tumor were evaluated with IHC using cathepsin B and MMP-13 antibodies. IHC results showed that the considerable amount of tumor cells were cathepsin B positive (Figure 6C). The cathepsin B expression and the location of cathepsin B probe signals in tumor tissue were coincided in tumor tissues (Figure 6D). Cathepsin B expression was not found in lung, spleen, and heart tissues, and the NIRF signal recovery of cathepsin B probes was not observed in these tissues, either (data not shown). However, the vascular endothelial cells including sinusoidal capillaries of the liver were cathepsin B positive in the IHC results (Figure 6E), while the cytoplasm of hepatocytes did not express cathepsin B. Regardless of the cathepsin B expression in blood vessels, the unexpected NIRF recovery was diffusely observed in the liver tissues including hepatocytes (Figure 6F).
In vivo NIRF tomographic images of subcutaneous SCC7 tumor-bearing mice after intravenous injection of cathepsin B and MMP probes. (A) The NIRF in the tumor site of the cathepsin B probe injected mouse. (B) The NIRF in the tumor site of the MMP probe injected mouse. (C) Comparison of the NIRF intensity at tumor site of cathepsin B and MMP probe-injected mice (p < .05).
NIRF signal of cathepsin B and MMP probes in excised SCC7 tumors and other visceral organs. (A) Representative ex vivo NIRF imaging of cathepsin B probe and MMPs injected tumor bearing mice scarified at fluorescence intensity peak time. (B) Comparison of the NIRF intensity of two probes in visceral organs and tumor. (C) Cathepsin B expression in the cytoplasm of the tumor cells (brown). Immunohistochemistry (IHC); horse radish peroxidase and diaminobenzidine (HRP-DAB)/hematoxylin. (D) NIRF of cathepsin B probe in tumor tissue (red). Fluorescence imaging; 695 nm. (E) Cathepsin B negative hepatocytes and cathepsin B stained vascular endothelia in liver. (F) NIRF of cathepsin B probe in liver tissues. (G) Diffusely stained tumor cells with MMP-13 antibody. (H) NIRF of MMP probe in tumor tissue (I) MMP-13 negative hepatocytes. (J) Low NIRF recovery of MMP probe in liver tissues.
The disagreement of cathepsin B expression and cathepsin B probe signals in liver tissues suggested that cathepsin B probe can be dequenched by non-specific factors. In tissue study, the cathepsin B probe showed relatively low specificity.
MMP-13 expression in tumor tissue was identified in the tumor cells diffusely (Figure 6G), and the MMP probe signal was detected in the tumor site with agreement (Figure 6H). The lung, kidney, spleen, heart, and liver (Figure 6I) did not express MMP-13, and dequenching of MMP probe by non-specific enzymatic activities was not observed in the liver tissue (Figure 6J). The MMP probe was different from the cathepsin B probe in probe activities in visceral organs including liver.
Discussion
Our MMP and cathepsin B probes showed an excellent sensitivity based on quenching/ dequenching system with fluorochrome dye (Cy 5.5) and dark quencher (BHQ-3). They showed significantly lower fluorescent backgrounds comparing to conventional FRET system. This is because BHQ-3 is a strong absorber of Cy-5.5 and has no native fluorescence, which is an efficient quencher of Cy5.5 for the development of NIRF probes [32]. Therefore, both strongly quenched probes could provide high contrast images by dequenching in response to target proteases at tumor. The dequenched cathepsin B probe and MMP probe showed up to 50.0- and 48.4-times higher fluorescence intensity than quenched one in vitro. Moreover, we confirmed that each probe showed a proportional relation between recovered fluorescence signal and different concentrations of corresponding protease (2, 4 and 8 nM) (r2=0.97).
Although both probes demonstrated superb specificity to cancer-related cathepsin B or MMPs, the dequenching of the probes by target protease was different depending on the biological environment. In cultured tumor cells, cathepsin B expression was clearly limited in the cytoplasm of SCC-7 cells. However, MMP-13 expression was observed in whole tumor cell including strong MMP-13 positive nuclei. Previously, Roebuck et al. also reported that MMP-13 expression was identified in the nuclei of the malignant tumor cells [33], and the MMP-13 observed in this study was consistent with the result. In spite of the different distribution of cathepsin B and MMP-13 in cultured SCC-7 cells, the NIRF recovery of each probe was dequenched by its target protease expression in cultured cells. The difference in the localization of NIRF signals may be due to the biological properties of the cathepsin B and MMP. Cathepsin B is the lysosomal protease in the cytoplasm, but MMPs are the mostly secreted protease. We further studied the activities of two probes in extracelluar milieu. The NIRF image of cell culture media reflects the ability to activate probe in extracelluar space [34]. Thus, the activities of two probes in culture supernatant were evaluated, and the significant recovery of the NIRF signals only occurred in the MMP probe treated cell culture supernatant. Even at the 12.5 nM of MMP probe concentration, MMP probe successfully showed fluorescence recovery by MMPs including MMP-13 in cell culture supernatant. Because cathepsin B is generally localized in the cytoplasm, the culture supernatant of SCC-7 cells contains little or no cathepsin B. Therefore, cathepsin B probe maintained in quenched state in cell culture supernatant, and the NIRF signals were recovered in the cytoplasm only after cellular uptake.
The obtained maximum NIRF intensity in tumor was observed at different time point. The peak fluorescence intensity of cathepsin B probe treated group was observed 3 hours later than MMP probe treated group, which revealed the location and dequenching time of the probe at the target site. The cathepsin B probe should be internalized into the cytoplasm and encounter the lysosomal enzymes to be dequenched, and these steps may require certain amount of time. Three hours of delay may be the time for the cathepsin B probe to internalize into the cells.
Another important difference was found in ex vivo imaging. Although the fluorescent recovery in non-tumor site was not significantly observed during in vivo NIRF imaging, the ex vivo NIRF imaging of cathepsin B probe injected mice showed unexpected fluorescence recovery in liver. While the cultured tumor cells are composed of only single type of cells, the excised tumor tissue samples contain multiple cell populations including stromal, endothelial, and normal epithelial cells. It is not simple to predict or interpret the dynamics of the probe in vivo, since the tumor cells are only the part of the total cell population of bulk tumor samples [35]. In addition, tumor-specificity of the probe in vivo may be decreased, if non-tumor cells in any other organs also produce the target proteases. Especially, liver generally produces various enzymes which can accidentally response to the probes. Liver also has an abundant blood supply and blood vessels including sinusoidal vessels. Therefore, the specificity of probes in vivo could be estimated by the comparison of fluorescence recovery in target site and liver. In this point of view, MMP probe showed relatively higher specificity in vivo compared to cathepsin B probes.
Non-specific fluorescence recovery of cathepsin B probe in liver was more distinct in tissue study. IHC results confirmed that both cathepsin B and MMP-13 were overexpressed in tumor, but not in the normal hepatocytes. Consequentially, MMP probe was dequenched in MMPs rich site of tumor tissue, but no NIRF signal recovery was observed in liver tissues. On the contrary, non-specific fluorescence recovery was observed in the liver tissue of cathepsin B probe injected mice without any histopathological changes of liver. The endothelial cells of sinusoidal vessels and hepatic veins were immunoreactive with cathepsin B antibody, but hepatocytes were all cathepsin B negative. It was not clear whether cathepsin B is expressed in hepatic vascular endotheila, or there are unknown molecules that are similar to the epitope of cathepsin B antibody in liver. Because of this non-specific dequenching of cathepsin B probe in liver, we concluded that the MMP probe has higher specificity than cathepsin B probe in ex vivo imaging and histopathology as well as in vivo condition.
Due to these different biological behaviors of probes depending on various biological environments, clinical application of the probes for cancer diagnosis and therapy would be discriminated from each other. Extracellular MMP can dequench the MMP probe even before the probe enter the cytoplasm, and enhanced permeability and retention effect of tumor vasculature permits accumulation and subsequent dequenching of the MMP probe. In other words, the MMP probe can exhibit fluorescence recovery immediately after reaching the tumor site without uptake into tumor cells. In contrast, cathepsin B probe can be dequenched to recover its fluorescence after the probe enters the cytoplasm. Based on this property, the cathepsin B probe can be used as a tool for evaluating the cytoplasmic delivery of the drugs or nanoparticles for cancer therapy in vivo. Intraveonously injected drugs or nanoparticles can be non-invasively analyzed by monitoring in vivo NIRF imaging after cathepsin B probe conjugation. The changes of NIRF intensity would be apparent after the cellular uptake of cathepsin B probe conjugated drugs or nanoparticles.
On the other hand, MMP probe showed higher specificity in vivo because it was not reactive in other visceral organs including liver. Consequently, MMP probe is more suitable to monitor whether probe conjugated -drug or -nanoparticle is delivered to the target tumor site or not. In addition, considering of biological functions of the proteases, MMP probe has another advantage of a good indicator for malignant tumor. Previous studies suggested that MMPs were closely related with malignant tumor [16, 17, 36], and the tumor-derived MMPs are intimately involved in high malignancy and poor prognoses of various cancers [37, 38]. Although both cathepsin B and MMPs are concerned in invasion and metastasis of cancer cells, MMPs and the cancer prognosis has been studied more intensively. Therefore, in cancer research, monitoring MMPs with this probe signals may provide more useful information related with cancer progression or predicting the prognosis.
Conclusion
In the present study, we compared the different dequenching conditions of cathepsin B probe and MMP probe depending on various biological environments related with cancer. Both probes utilized quenched fluorescence with protease-substrate peptides to show good specificity in tumor, but each probe showed its own advantages depending on individual conditions. MMP probe demonstrated superior specificity in ex vivo imaging and histology, and it would be useful for the study of malignant tumor and its metastasis. Cathepsin B probe were useful for evaluating the cytoplasmic targeted delivery, because it was dequenched in the cytoplasm only. With the cathepsin B probe, the practical delivery of anti-cancer drugs or drug loaded nanoparticles into the cytoplasm would be confirmed effectively. This study provides valuable information about the activities of two probes in various conditions including cultured cell, culture supernatant, in vivo, and tissues. Understanding the biological functions and biological properties of each probe will allow a better use and proper application of the probes in cancer theragnosis.
Acknowledgements
This research was supported by Fusion Technology Project (2009-0081876) of MEST and the Intramural Research Program (Theragnosis) of KIST.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Levi J, Kothapalli SR, Ma TJ, Hartman K, Khuri-Yakub BT, Gambhir SS. Design, synthesis, and imaging of an activatable photoacoustic probe. J Am Chem Soc. 2010;132:11264-9
2. Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168-71
3. Kim GB, Kim Y. Analysis of protease activity using Quantum dots and resonance energy transfer. Theranostics. 2012;2:127-138
4. Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3:668-77
5. Zheng G, Chen J, Stefflova K, Jarvi M, Li H, Wilson BC. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc Natl Acad Sci U S A. 2007;104:8989-94
6. Lee S, Ryu JH, Park K, Lee A, Lee SY, Youn IC. et al. Polymeric nanoparticle-based activatable near-infrared nanosensor for protease determination in vivo. Nano Lett. 2009;9:4412-6
7. Ryu JH, Kim SA, Koo H, Yhee JY, Lee A, Na JH. et al. Cathepsin B-sensitive nanoprobe for in vivo tumor diagnosis. J Mater Chem. 2011;21:17631-4
8. Law B, Curino A, Bugge TH, Weissleder R, Tung CH. Design, synthesis, and characterization of urokinase plasminogen-activator-sensitive near-infrared reporter. Chem Biol. 2004;11:99-106
9. Zhu L, Xie J, Swierczewska M, Zhang F, Quan Q, Ma Y. et al. Real-time video imaging of protease expression in vivo. Theranostics. 2011;1:18-27
10. Lu J, Zhang Z, Yang J, Chu J, Li P, Zeng S. et al. Visualization of beta-secretase cleavage in living cells using a genetically encoded surface-displayed FRET probe. Biochem Biophys Res Commun. 2007;362:25-30
11. Lin J, Zhang Z, Yang J, Zeng S, Liu BF, Luo Q. Real-time detection of caspase-2 activation in a single living HeLa cell during cisplatin-induced apoptosis. J Biomed Opt. 2006;11:024011
12. Chen Y, Liang G. Enzymatic Self-Assembly of Nanostructures for Theranostics. Theranostics. 2012;2(2):139-147
13. Jang B, Choi Y. Photosensitizer-conjugated Gold Nanorods for Enzyme-Activatable Fluorescence Imaging and Photodynamic Therapy. Theranostics. 2012;2(2):190-197
14. Zucker S. A critical appraisal of the role of proteolytic enzymes in cancer invasion: emphasis on tumor surface proteinases. Cancer Invest. 1988;6:219-31
15. Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000;291:113-35
16. Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG. et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683-93
17. Ala-aho R, Kahari VM. Collagenases in cancer. Biochimie. 2005;87:273-86
18. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-74
19. Yamada T, Oshima T, Yoshihara K, Tamura S, Kanazawa A, Inagaki D. et al. Overexpression of MMP-13 gene in colorectal cancer with liver metastasis. Anticancer Res. 2010;30:2693-9
20. Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437-44
21. Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T. et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001;84:1488-96
22. Hulkower KI, Butler CC, Linebaugh BE, Klaus JL, Keppler D, Giranda VL. et al. Fluorescent microplate assay for cancer cell-associated cathepsin B. Eur J Biochem. 2000;267:4165-70
23. Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800-8
24. Ebert MP, Kruger S, Fogeron ML, Lamer S, Chen J, Pross M. et al. Overexpression of cathepsin B in gastric cancer identified by proteome analysis. Proteomics. 2005;5:1693-704
25. Kruszewski WJ, Rzepko R, Wojtacki J, Skokowski J, Kopacz A, Jaskiewicz K. et al. Overexpression of cathepsin B correlates with angiogenesis in colon adenocarcinoma. Neoplasma. 2004;51:38-43
26. Kawada A, Hara K, Kominami E, Kobayashi T, Hiruma M, Ishibashi A. Cathepsin B and D expression in squamous cell carcinoma. Br J Dermatol. 1996;135:905-10
27. Lorenzo K, Ton P, Clark JL, Coulibaly S, Mach L. Invasive properties of murine squamous carcinoma cells: secretion of matrix-degrading cathepsins is attributable to a deficiency in the mannose 6-phosphate/insulin-like growth factor II receptor. Cancer Res. 2000;60:4070-6
28. Pham W, Choi Y, Weissleder R, Tung CH. Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjug Chem. 2004;15:1403-7
29. van Duijnhoven SM, Robillard MS, Nicolay K, Grull H. Tumor targeting of MMP-2/9 activatable cell-penetrating imaging probes is caused by tumor-independent activation. J Nucl Med. 2011;52:279-86
30. Ryu JH, Lee A, Huh MS, Chu J, Kim K, Kim BS, Choi K, Kwon IC, Park JW, Youn I. Measurement of MMP Activity in Synovial Fluid in Cases of Osteoarthritis and Acute Inflammatory Conditions of the Knee Joints Using a Fluorogenic Peptide Probe-Immobilized Diagnostic Kit. Theranostics. 2012;2(2):198-206
31. Koo H, Lee H, Lee S, Min KH, Kim MS, Lee DS. et al. In vivo tumor diagnosis and photodynamic therapy via tumoral pH-responsive polymeric micelles. Chem Commun (Camb). 2010;46:5668-70
32. Koo H, Huh M.S, Ryu J.H, Lee D.-E, Sun I.-C, Choi K, Kim K, Kwon I.C. Nanoprobes for biomedical imaging in living systems. Nano Today. 2011;6:201-20
33. Roebuck MM, Helliwell TR, Chaudhry IH, Kalogrianitis S, Carter S, Kemp GJ. et al. Matrix metalloproteinase expression is related to angiogenesis and histologic grade in spindle cell soft tissue neoplasms of the extremities. Am J Clin Pathol. 2005;123:405-14
34. Sun IC, Eun DK, Koo H, Ko CY, Kim HS, Yi DK. et al. Tumor-targeting gold particles for dual computed tomography/optical cancer imaging. Angew Chem Int Ed Engl. 2011;50:9348-51
35. Emmert-Buck MR, Roth MJ, Zhuang Z, Campo E, Rozhin J, Sloane BF. et al. Increased gelatinase A (MMP-2) and cathepsin B activity in invasive tumor regions of human colon cancer samples. Am J Pathol. 1994;145:1285-90
36. Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990;1:99-106
37. Zhang B, Cao X, Liu Y, Cao W, Zhang F, Zhang S. et al. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer. 2008;8:83
38. Holtkamp N, Atallah I, Okuducu AF, Mucha J, Hartmann C, Mautner VF. et al. MMP-13 and p53 in the progression of malignant peripheral nerve sheath tumors. Neoplasia. 2007;9:671-7
Author contact
![]() Corresponding author: Kwangmeyung Kim, PhD, KIST, Biomedical Research Center, Haweolgog-Dong, Sungbook-Gu, Seoul 136-791, Korea. Tel: +82 2 958 5916; Fax: +82 2 958 5909. E-mail: kimre.kr
Corresponding author: Kwangmeyung Kim, PhD, KIST, Biomedical Research Center, Haweolgog-Dong, Sungbook-Gu, Seoul 136-791, Korea. Tel: +82 2 958 5916; Fax: +82 2 958 5909. E-mail: kimre.kr
 Global reach, higher impact
Global reach, higher impact