13.3
Impact Factor
Theranostics 2011; 1:48-57. doi:10.7150/thno/v01p0048 This volume Cite
Review
PET Imaging of Integrin αVβ3 Expression
1. Department of Nuclear Medicine, Klinikum rechts der Isar, Technische Universität München, Munich, Germany
2. Institute for Advanced Study und Center of Integrated Protein Science, Technische Universität München, Department Chemie, Garching, Germany
3. Chair for Pharmaceutical Radiochemistry, Garching, Germany
Published 2011-1-17
Abstract
PET imaging of integrin αvβ3 expression has been studied intensely by the academia and recently also by the industry. Imaging of integrin αvβ3 expression is of great potential value, as the integrin αvβ3 is a key player in tumor metastasis and angiogenesis. Therefore PET imaging of this target might be a suitable in-vivo biomarker of angiogenesis and metastatic potential of tumors. In this manuscript, the various strategies for PET imaging of the integrin αvβ3 will be summarized, including monomeric and multimeric radiolabelled RGD peptides and nanoparticles. While most experiments have been performed using preclinical tumor models, more and more clinical results on PET imaging of αvβ3 expression are available and will be discussed in detail. However, while a multitude of radiotracer strategies have been successfully evaluated for PET imaging of αvβ3, the ultimate clinical value of this new imaging biomarker still has to be evaluated in large clinical trials.
Keywords: PET, integrin αvβ3, molecular imaging, angiogenesis, metastasis
1. INTRODUCTION
Integrins are heterodimeric transmembrane glycoproteins consisting of different α- and β-subunits which play an important role cell-cell- and cell-matrix-interactions. Especially well examined is the integrin αvβ3 and its role in angiogenesis and tumor metastasis by facilitating endothelial and tumor cell migration. Angiogenesis is a fundamental process involved in a variety of physiological as well as pathological conditions. Physiologically, it is required for development, wound repair, reproduction and response to ischemia. Pathologically, it is associated with disease conditions like arthritis, psoriasis, retinopathies and cancer [1]. Since Folkman in 1971 first articulated the concept that the growth of solid tumors remains restricted to 2-3 mm in diameter until the onset of angiogenesis, subsequent investigations have identified more than 20 angiogenic growth factors, their receptors and signal transduction pathways. Moreover, endogenous angiogenesis inhibitors have been discovered and the cellular and molecular characterization of the angiogenic phenotype in human cancers has been achieved [2]. The concept of antiangiogenic therapy has evolved over the last years as a therapeutic strategy in clinical oncology aimed at stopping cancer progression by suppressing the tumor blood supply. Especially, the VEGF (vascular endothelial growth factor) antibody Avastin® has shown favourable results in combination with standard cytotoxic chemotherapy in metastasized colorectal cancer, breast cancer and non-small cell lung cancer [3-4]. This increasing use of targeted therapies leads to a growing demand for imaging tumor response to these therapies, as usually only a subset of patients will respond to these very specific drugs. However, as antiangiogenetic agents rather lead to a stop of tumor progression than to tumor shrinkage, the approach of measuring tumor response by a reduction of tumor size is not applicable and might take a very long time to assess. Therefore, there is great need for imaging biomarkers of early tumor response to noncytotoxic drugs, which predict subsequent clinical response [5]. Such biomarkers would not only facilitate clinical trials of new drugs but could also be used to aid in the selection of optimal treatment for individual patients (“personalized medicine”). Positron emission tomography (PET) using tracers for assessment of glucose metabolism by [18F]FDG or proliferation by [18F]FLT or qualitative assessment of sst-receptor expression by [68Ga]DOTATOC has already shown promising results in clinical studies for response assessment of cytotoxic chemotherapies and peptide receptor radiotherapy [6-7]. In a similar way, targeting specific molecular markers of angiogenesis by PET imaging might be used for response assessment of antiangiogenic therapies, like the integrin αvβ3. In this chapter we will discuss the different strategies currently in use for imaging of integrins with PET. As most published results deal with imaging of the integrin αvβ3 and as this is the only integrin to date successfully visualized in patients with PET, we will focus on this target.
2. CHARACTERISTICS OF PET IMAGING
PET imaging has the advantage of being very sensitive to low concentrations of tracer molecules and of having unlimited depth penetration. PET is approximately 10 times more sensitive than SPECT and is able to detect very low amounts of tracers at the picomolar range. Moreover, PET is a quantitative imaging modality which is an advantage over MRI and conventional optical imaging techniques. However, with the introduction of fluorescence mediated tomography (FMT), quantitative measurements are also possible with OI techniques [8]. PET is well suited to molecular imaging of angiogenesis using targeted tracers because of the generally low concentrations of target molecules. This also facilitates translation of preclinical results and tracers to the clinic, because toxicity is rarely an issue with PET tracers due to the low absolute amount of substance used. This is e.g. advantageous compared to classical MR contrast agents, where usually substantially larger amounts of substances have to be used. However, PET usually can only be performed at facilities that have the necessary cyclotron or have a cyclotron nearby for delivery of radiotracers and which the required radiochemical laboratories for the preparation of the tracers. On the contrary, SPECT imaging is much more widely available than PET imaging and the radionuclides used for SPECT are easier to prepare and usually have a longer half-life than those used for PET (6 hours for [99mTc], 67 hours for [111In], and 13.2 hours for [123I]). However, with the increasing use of generator produced positron emitting radionuclides like [68Ga], this problem might be overcome in the future for PET as well [9]. Another limitation of PET is the lower spatial resolution compared to MRI, CT and US. With current state of the art clinical PET scanners, a resolution of 3-4 mm is possible [10]. However, this does not apply to preclinical animal scanners, which may allow for submillimeter resolutions even in small rodents [11].
3. PET IMAGING OF INTEGRIN αvβ3 EXPRESSION
In the clinics, the most commonly used way to assess vascularisation and angiogenesis is the use of functional imaging, e.g. dynamic contrast enhanced MRI or CT (DCE MRI / DCE CT). While functional imaging of hemodynamic parameters of angiogenesis can be performed on routinely used MR and CT scanners, the interpretation of the results with regard to their physiological meaning often remains difficult [12]. Moreover, most of the methods applied are technically challenging and with regard to DCE MRI difficult to standardize [13]. Therefore more specific markers of angiogenetic activity in tumors are desirable to facilitate assessment of angiogenesis and response evaluation during antiangiogenic therapy. One approach is to identifying molecular markers of and to use specific ligands to these targets conjugated with positron emitters which allow for PET imaging. As mentioned before, one of the most promising and best examined targets in this respect is the integrin αvβ3, which has been studied extensively as a target for molecular imaging preclinically, but also has been studied in first clinical studies in the past years [14].
Integrins are heterodimeric transmembrane glycoproteins consisting of different α- and β-subunits which play an important role cell-cell- and cell-matrix-interactions and tumour metastasis by facilitating endothelial and tumor cell migration. It has been found that several extracellular matrix (ECM) proteins like vitronectin, fibrinogen and fibronectin interact with integrins via the amino acid sequence arginine-glycine-aspartic acid or RGD in the single letter code [15]. Based on these findings, monomeric as well as multimeric linear as well as cyclic peptides including the RGD sequence have been introduced. The pentapeptide cyclo(-Arg-Gly-Asp-DPhe-Val-) was developed by Kessler and co-workers and shows high affinity and selectivity for αvβ3 [54]. It has evolved as one of the most prominent lead structures for the development of molecular imaging compounds for the assessment of αvβ3 expression [16, 55]. For the first evaluation of this approach, radioiodinated RGD peptides have been synthesised which showed comparable affinity and selectivity to the lead structure. In vivo they revealed receptor-specific tumour uptake but also predominantly hepatobiliary elimination, resulting in high activity concentration in liver and intestine, which is unfavourable for patient studies [17]. Several strategies to improve the pharmacokinetics of radiohalogenated peptides have therefore been developed. These strategies comprise conjugation with sugar moieties, hydrophilic amino acids and polyethylene glycol (PEG). The glycosylation approach is based on the introduction of sugar derivatives which are conjugated to the ε-amino function of a corresponding lysine in the peptide sequence. By conjugating the RGD containing cyclic pentapeptide cyclo(-Arg-Gly-Asp-DPhe-Val-) with glucose- or galactose-based sugar amino acids, [*I]Gluco-RGD and [18F]Galacto-RGD have been developed for PET and SPECT imaging (Fig. 1).
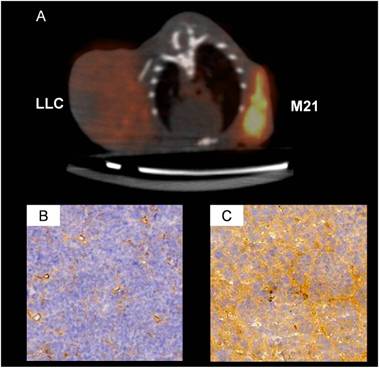
Example of a [18F]Galacto-RGD microPET scan of a nude mouse (A) with a Lewis Lung Cell Cancer (LLC) and a M21 melanoma xenograft (M21). Corresponding immunohistochemistry of αvβ3 expression is shown for the LLC in B and for the M21 tumour in C. The LLC tumor shows αvβ3 expression on the neovasculature but not on the tumor cells, while the M21 tumour shows αvβ3 expression both on the neovasculature and on the tumor cells. Consequently, the PET signal is much more intense in the M21 tumour and only moderate in the LLC tumor.
The glycosylation strategy improved pharmacokinetics of these compounds, which showed predominantly renal tracer elimination and increased activity uptake and retention in a murine tumour model compared with the first generation peptides [18]. The conjugation of hydrophilic D-amino acids was also used to improve the pharmacokinetics of peptide-based tracers [19]. Again, tracer elimination could be shifted to the renal pathway, but tumour uptake of the compound [18F]DAsp3-RGD was lower than that found for [18F]Galacto-RGD. However, tumour/background ratios calculated from small animal PET images were comparable due to the even faster elimination. PEGylation is another way to improve many properties of peptides and proteins [20]. Chen et al. conjugated RGD-containing peptides with PEG moieties with different sizes, using different radiolabelling strategies. These studies revealed very different effects of PEGylation on the pharmacokinetics and tumour uptake and retention of RGD peptides, which seem to depend strongly on the nature of the lead structure and perhaps on the size of the PEG moiety [21].
Extensive preclinical evaluations concerning monomeric compounds has been carried out using [*I]Gluco-RGD and [18F]Galacto-RGD [22-23]. The M21human melanoma model is well characterized concerning αvβ3 expression and has been used for initial in vivo evaluation [24]. Using this model, [18F]Galacto-RGD and [125I]Gluco-RGD uptake in the tumour 120 min p.i. was 1.5 and 1.8% ID/g, respectively. Blocking experiments injecting 6 mg c(RGDfV) per kg mouse 10 min prior to tracer injection reduced tumour accumulation to approximately 15% of control for [125I]Gluco-RGD and to approximately 35% of control for [18F]Galacto-RGD, which demonstrates receptor specific accumulation. Furthermore, imaging studies with mice bearing melanoma tumours with increasing amounts of αvβ3-positive cells (produced by mixing M21 and M21-L cells) showed that there is a correlation between integrin expression and tracer accumulation [14]. These data demonstrate that non-invasive determination of αvβ3 expression and quantification with radiolabelled RGD peptides is feasible even when using static emission scans. This is an important aspect for future clinical use, as static emission scanning is easier to perform than dynamic scanning and moreover allows for whole-body imaging, whereas dynamic imaging is limited to one bed position of the scanner. Moreover, animal PET studies with increasing amounts of c(RGDfV) were obtained that indicated that the dose-dependent blocking of tracer uptake in the receptor positive tumour could be monitored. Additional experiments were carried out with the RIPTag model, in order to assess, whether serial imaging with radiolabelled RGD-peptides allows for determination of the time point, when angiogenic activity begins, or the time point of the “angiogenic switch”. The RIPTag model is a transgenic mouse model of carcinogenesis and angiogenesis where the oncogene SV40 T antigen is expressed under the control of the rat insulin promoter [25]. Immunohistochemical staining of pancreatic sections with a monoclonal antibody against the murine β3 subunit indicated that αvβ3 is exclusively expressed on the vessels of the insulinoma and not on the tumour cells themselves. Autoradiographic studies of pancreatic sections of mice sacrificed 2 h after i.v. injection of [125I]Gluco-RGD showed high focal activity accumulation in the pancreas in RIP-Tag-positive mice. In contrast, for the RIP-Tag-negative mice only low activity accumulation corresponding to the background was found. Moreover, there was a clear increase in activity accumulation in the pancreas of RIP-Tag-positive mice between 7 and 9 weeks, which corresponds with the tumour differentiation and the time point of the angiogenic switch. In contrast, for RIP-Tag-negative mice, tumour accumulation remained low over the whole observation period. In conclusion, these data suggest that αvβ3 expression can be quantified by radiolabelled RGD-peptides using static emission scans, and that αvβ3 expression on endothelial cells can be monitored sequentially during tumour-induced angiogenesis with radiolabelled RGD-peptides.
However, lesion tracer uptake of many monomeric compounds can still be optimized because lesion identification is difficult in areas with high physiological tracer uptake of monomeric RGD peptides, such as liver, spleen and intestine.
Variations in tracer design are supposed to further improve the performance of αvβ3 imaging, e.g. using multimeric RGD peptides. Since the interaction between integrin αvβ3 and RGD-containing ECM-proteins involves multivalent binding sites with clustering of integrins, the concept to improve the integrin αvβ3 binding affinity with multimeric cyclic RGD peptides could provide more effective antagonists with better targeting capability and higher cellular uptake through the integrin-dependent endocytosis pathway [26,57,58]. A series of RGD peptides have been labelled with [18F] for PET imaging by Chen et al., using PEGylation and polyvalency to improve the tumor-targeting efficacy and pharmacokinetics. [18F]FB-E[c(RGDyK)]2 (abbreviated as [18F]FRGD2) showed predominantly renal excretion and almost twice as much tumor uptake in the same animal model compared with the monomeric tracer [18F]FB-c(RGDyK) [27-28]. Linear regression analysis of the dynamic microPET scans in six tumor xenograft models was carried out to correlate the tumor uptake with integrin αvβ3 expression level measured by SDS-PAGE autoradiography, and showed an excellent correlation. Moreover, at late time points when most of the nonspecific binding had been cleared, the tumor/background ratio had a linear relationship with tumor integrin αvβ3 expression. This demonstrates that quantification of αvβ3 expression is also feasible with static emission scans, which facilitates translation into the clinic. Multimeric RGD peptides have also been labeled with [64Cu] for PET imaging, using PEGylation and a multimeric approach to optimize the tumor-targeting efficacy and pharmacokinetics [29-31]. The tetrameric RGD peptide-based tracer, [64Cu]DOTA-E[E[c(RGDfK)]2]2, showed significantly higher receptor binding affinity than the corresponding monomeric and dimeric RGD analogues and demonstrated rapid blood clearance, high metabolic stability, predominant renal excretion and significant receptor-mediated tumor uptake with good contrast in xenograft-bearing mice [32]. Therefore, [64Cu]DOTA-E[E[c(RGDfK)]2]2 is a promising agent for peptide receptor radionuclide imaging as well as targeted internal radiotherapy of αvβ3-positive tumors. A RGD octamer even further increased the integrin avidity by another 3-fold compared with tetramer. In vivo microPET imaging showed that [64Cu]DOTA-RGD octamer had slightly higher initial tumor uptake and much longer tumor retention in U87MG tumor that express high level of integrin. However, the octamer exhibited significantly higher tumor uptake in mammary adenocarcinoa-bearing c-neu oncomice that express medium level of integrin. The high renal uptake of the octamer in both subcutaneous U87MG xenografts and mammary adenocarcinoma-bearing c-neu oncomice compared with the tetramer was attributed mainly to the integrin positivity of the kidneys [33]. A systematic study on the influence of multimerisation on receptor affinity and tumour uptake was carried out by the groups of Wester and Kessler who synthesised a series of monomeric, dimeric, tetrameric and octameric RGD peptides. These compounds contain different numbers of c(RGDfE) peptides connected via PEG linker and lysine moieties, which are used as branching units. They found an increasing binding affinity in the series monomer, dimer, tetramer and octamer in an in vitro binding assay, which was confirmed by small animal PET studies. Moreover, PET studies comparing a tetrameric structure containing four c(RGDfE) peptides with a tetrameric compound containing only one c(RGDfE) and three c(RaDFE) peptides, which do not bind to the αvβ3 integrin, showed a threefold lower activity accumulation in the tumour for the pseudo monomeric tetramer than for the “real” tetramer, indicating that the higher uptake in the tumour really is due to multimerisation and not based on other structural effects [34]. Furthermore they could demonstrate that moderate metabolization of multimeric constructs linked with L-Lys residues can improve tumor/background ratios when compared to analogues linked with metabolically stable D-Lys residues.
Overall, the multimerisation approach leads to increased binding affinity and tumour uptake as well as retention and can improve the pharmacokinetics of peptide-based tracers. However, this does not necessarily has to relate to better tumor-to-background contrast or improved clinical performance. A recent comparison of the monomeric compound [18F]Galacto-RGD and a dimeric RGD-peptide showed similar tumor-to-background contrast, despite higher absolute uptake of the dimeric compound in the tumor [35]. Still, multimeric RGD peptides hold a lot of promise for future clinical use and first results of human studies are eagerly awaited.
Another strategy to image αvβ3 expression by PET is to use radiolabelled nanoparticles. In general, the purpose of nanoparticle-based radiotracers for αvβ3 imaging is a little different from previously described peptide- or antibody- based imaging. The focus of imaging with nanoparticle-based radiotracers is to provide guidance for integrin targeted drug delivery or therapy and not necessarily to evaluate receptor expression levels. Cai et al. recently developed a QD-based probe for both NIRF and PET imaging [36]. QD surface modification with RGD peptides allows for integrin αvβ3 targeting and DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; a very effective chelator for many metal ions) conjugation enables PET imaging after [64Cu]-labeling. Using this dual-modality probe, it was found that the majority of the probe in the tumor was within the tumor vasculature.
Another nanoparticle-approach is the use of single-walled carbon nanotubes (SWNTs). SWNTs exhibit unique size, shape, and physical properties that make them promising candidates for biological applications [37-38]. Liu et al. recently investigated the biodistribution of [64Cu]-labeled SWNTs in mice by PET, biodistribution, and ex vivo Raman spectroscopy [39]. It was found that properly PEGylated SWNTs have relatively long circulation half-life (a few hours) and low uptake by the reticuloendothelial system (RES). Efficient targeting of integrin αvβ3-positive U87MG tumor in mice (~ 15 %ID/g), among the highest of any nanoparticles ever reported, was also achieved with RGD peptide conjugated SWNTs. The unique Raman signatures of SWNTs enabled direct measurement of SWNTs in various mice tissue which confirmed the radionuclide-based results. Virtually no kidney uptake was observed based on Raman measurement of the tissue homogenate, although a small fraction of [64Cu] detached from the SWNT did give appreciable kidney uptake in PET imaging. SWNTs have the advantage of a comparably large surface area that can be potentially functionalized in a variety of ways to attach therapeutic agents and other moieties, for integrated multimodality imaging and molecular therapy [36].
Up to now, the radiotracer approach is still the only technique for imaging αvβ3 expression that has made the transition into the clinic. [18F]Galacto-RGD was the first PET tracer applied in patients and could successfully image αvβ3 expression in human tumors with good tumor/background ratios. In all patients, rapid, predominantly renal tracer elimination was observed, resulting in low background activity in most regions of the body [40]. High inter- and intra-individual variance in tracer accumulation in tumor lesions was noted, suggesting substantial heterogeneity of αvβ3 expression (Fig. 2). Further biodistribution and dosimetry studies have confirmed rapid clearance of [18F]Galacto-RGD from the blood pool and primarily renal excretion. Background activity in lung and muscle tissue was low and the calculated effective dose found was approximately 19 μSv/MBq, which is very similar to an [18F]FDG scan [41]. Therefore this tracer can be safely used in the clinic. Distribution volume (Dv) values, which are supposed to reflect the receptor concentration in the tissue, were on average four times higher for tumour tissue than for muscle tissue, suggesting specific tracer binding. In a recent study, dynamic emission scans over 60 minutes and kinetic modelling studies using the aorta as arterial input function were performed in patients with invasive ductal breast cancer.
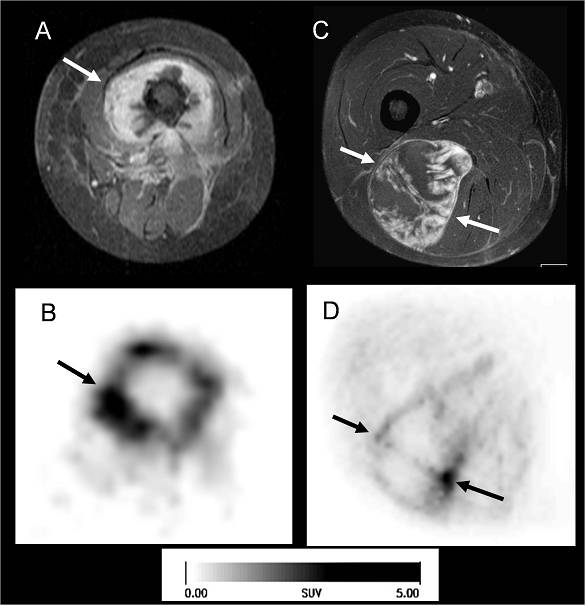
Two patients with sarcomas of the thigh (arrows). On the left side a patient with osteosarcoma of the right femur (A, B), on the right side a patient with a liposarcoma (C, D). In the MRI (A, C: T1w contrast enhanced, with fat suppression; transaxial) you see the large tumors with intense contrast enhancement. Despite the similar patterns of contrast enhancement, the corresponding [18F]Galacto-RGD PET (B, D transaxial slices) shows quite different uptake patterns in both patients: in the osteosarcoma we see intense tracer uptake, predominantly in the tumor periphery, whereas in the liposarcoma there is mostly faint tracer uptake, with some focal areas of more intense tracer uptake. This suggests very heterogeneous αvβ3 expression in these two tumors, despite intense contrast enhancement of both lesions.
We compared SUVs derived from the last 9 time frames with the Dv values measured in normal tissue (breast, muscle) and in the tumors. The correlation between both parameters increased continuously over time and was best at ~ 55 minutes p.i. with r = 0.92. This suggests that SUVs derived from static emission scans at ~ 60 min. p.i. can be used for assessment of αvβ3 receptor density with reasonable accuracy. Scans should not be started much earlier, as unspecific effects like tracer activity in the blood pool are likely to affect the SUVs. Finally, in another study, [18F]Galacto-RGD uptake was correlated with αvβ3 expression as determined by immunohistochemistry. 19 patients with solid tumours (musculoskeletal system n = 10, melanoma n = 4, head and neck cancer n = 2, glioblastoma n = 2, breast cancer n = 1) were examined with PET using [18]Galacto-RGD before surgical removal of the lesions [42]. SUVs and tumour/blood ratios correlated significantly with the intensity of immunohistochemical staining as well as with the microvessel density. Moreover, immunohistochemistry confirmed lack of αvβ3 expression in normal tissue and in the two tumours without tracer uptake. We have also systematically examined different tumor entities with respect to their αvβ3 expression patterns with [18F]Galacto-RGD PET. In squamous cell carcinoma of the head and neck (SCCHN) we demonstrated good tumor/background ratios with [18F]Galacto-RGD PET, with again a widely varying intensity of tracer uptake. Immunohistochemistry demonstrated predominantly vascular αvβ3 expression, suggesting that in SCCHN, [18F]Galacto-RGD PET might be used as a surrogate parameter of angiogenesis [43]. In patients with glioblastoma, results were more complex. While normal brain tissue did not show significant tracer accumulation (mean SUV, 0.09 +/- 0.04), GBMs demonstrated significant but heterogeneous tracer uptake, with a maximum in the highly proliferating and infiltrating areas of tumors (mean SUV, 1.6 +/- 0.5). Immunohistochemical staining was prominent in tumor microvessels as well as glial tumor cells. In areas of highly proliferating glial tumor cells, tracer uptake (SUVs) in the PET images correlated with immunohistochemical αvβ3 integrin expression of corresponding tumor samples [44]. However, tracer uptake was substantially lower as compared to results in tumors outside the CNS. As [18F]Galacto-RGD does not cross the intact blood-brain barrier, this might be an important factor influencing tracer uptake in CNS lesions. Therefore results obtained in the CNS and outside the CNS probably have to be interpreted differently.
We have also studied and compared the uptake patterns of [18F]FDG and [18F]Galacto-RGD in cancer patients with non small cell lung cancer (NSCLC, n=10) and various other tumors (n=8). This comparison is interesting because in case of a close correlation of the two tracers, there would probably be no need for a specific tracer like [18F]Galacto-RGD. The results showed no correlation between the two tracers concerning all lesions (r = 0.157). For the subgroup of [18F]FDG-avid lesions and lesions in patients with NSCLC, there was a slight trend towards a higher [18F]Galacto-RGD uptake in more [18F]FDG-avid lesions (r = 0.337). However, the correlation coefficient was very low. Our results suggest that αvβ3 expression and glucose metabolism are not closely correlated in tumour lesions and that consequently [18F]FDG cannot provide similar information as [18F]Galacto-RGD [45]. Currently we are correlating functional imaging of angiogenesis with DCE-MRI and molecular imaging of αvβ3 expression and glucose metabolism with [18F]Galacto-RGD and [18F]FDG in cancer patients. The combination of functional and molecular imaging holds great promise for non-invasive phenotyping of tumour biology and imaging of tissue heterogeneity in tumours. By dedicated imaging software we defined regions with different uptake patterns of [18F]Galacto-RGD and [18F]FDG: areas with both high [18F]Galacto-RGD and high [18F]FDG uptake (RGD+/FDG+), areas with either high [18F]Galacto-RGD and low [18F]FDG uptake (RGD+/FDG-) or vice versa (RGD-/FDG+), and finally areas with both low [18F]Galacto-RGD and low [18F]FDG uptake (RGD-/FDG-). In areas with both low [18F]Galacto-RGD and low [18F]FDG uptake, parameters of tissue perfusion as measured by DCE-MRI were significantly lower as compared to areas with both high [18F]Galacto-RGD and high [18F]FDG uptake. However, in areas with either high [18F]Galacto-RGD and low [18F]FDG uptake or vice versa, there was a broad overlap of DCE-MRI results. Regarding different tumor regions, a significant correlation between the initial area under the gadolinium concentration time curve vs. [18F]Galacto-RGD-PET (r=0.41; p<0.05) and all functional MRI parameters (IAUGC, regional blood flow and blood volume) vs. [18F]FDG-PET (r=0.37-0.54; p<0.05) could be demonstrated, however, with only low to moderate correlation coefficients [46]. These data suggest that in active tumor areas, perfusion, αvβ3 expression and glucose metabolism are largely independent variables of tumor biology. In summary multimodality functional and molecular imaging can provide complementary information and might help to evaluate tumor biology and angiogenesis in-vivo in its full complexity.
Despite the encouraging and promising results of [18F]Galacto-RGD, its rather complex synthesis hampers its broad use in large clinical studies. However, besides academia, industry has also shown interest in integrin imaging, which might facilitate PET imaging of integrin expression in large scale clinical trials, and maybe one day even in clinical routine. Recently, the SPECT tracer [99mTc]NC100692 was introduced by GE healthcare for imaging αvβ3 expression in humans and was first evaluated in breast cancer by Bach-Gansmo et al. 19 of 22 tumors could be detected with this agent, which was safe and well tolerated by the patients [47]. Moreover, also a PET imaging agent was introduced, called [18F]fluciclatide (or formerly [18F]AH111585). In an animal model of Lewis Lung Cell Cancer (LLC), PET imaging with [18F]AH111585 was able to visualize reduction of microvessel density during low dose paclitaxel therapy in an animal model, while uptake of [14C]FDG did not decrease [48]. This demonstrates that [18F]AH111585 might be of potential value for assessment of response to antiangiogenic therapy. First studies in humans have demonstrated favourable biodistribution of this tracer with predominantly renal excretion. Organs with highest radiation doses were the urinary bladder wall (0.124 mSv/MBq), the kidneys (0.102 mSv/MBq) and the cardiac wall (0.059 mSv/MBq). The mean effective dose was reported to be 0.026 mSv/MBq for 3.5 h urinary bladder voiding intervals [49]. In 7 patients with metastasized breast cancer all 18 tumors detected by CT were visible on the [18F]AH111585 PET images.
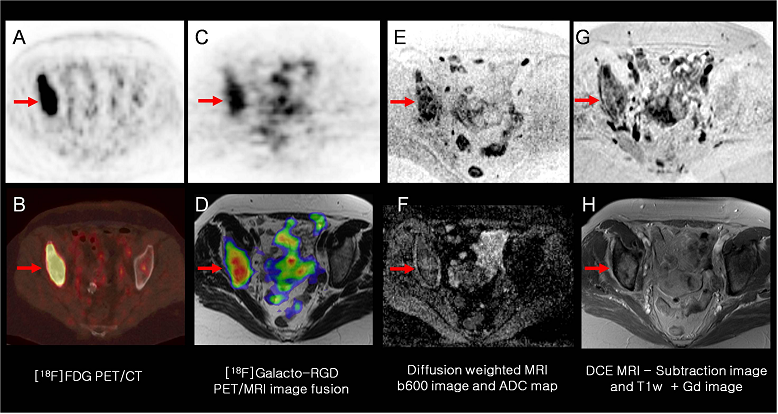
Example of multimodality multiparametric imaging of tumor biology in a patient with a metastasis of lung cancer to the right acetabulum (arrows). The [18F]FDG PET/CT (A: PET; B: PET/CT) shows intense tracer uptake in the lesion. [18F]Galacto-RGD PET (C: PET; D: PET/MRI image fusion) also shows intense tracer uptake, suggesting elevated αvβ3 expression in the lesion. Diffusion weighted MRI (E: b600 image, F: ADC map) shows restricted water diffusivity in the lesion as a potential marker of tumor cellularity. Finally DCE-MRI (G: subtraction image; H: contrast-enhanced T1w image) shows intense contrast enhancement of the lesion.
However, in the case of liver metastases, lesions were identified only indirectly as photopenic regions because of the high background activity in normal liver tissue. Increased uptake compared with background (p = 0.002) was demonstrated in metastases in lung, pleura, bone, lymph node, and primary tumor [50]. The authors concluded that [18F]AH111585 is safe, metabolically stable, and able to detect breast cancer lesions by PET in most anatomic sites.
Currently, a phase II study in up to 30 patients is being performed in patients with brain tumors, lung cancers, SCCHN, differentiated thyroid carcinoma, sarcoma and melanoma to correlate dynamic and static [18F]AH111585 PET imaging with histologic parameters of angiogenesis (including αvβ3 expression) and DCE-CT [51]. Based on these promising results, it is expected that commercial agents for PET imaging of αvβ3 expression might be available in the not too distant future.
4. PERSPECTIVE
PET imaging of integrin expression has successfully made its way from bench to bedside. A variety of PET tracers developed by the academia as well as the industry is currently being evaluated in clinical trials or in first dosimetry studies in patients [52]. Therefore a more widespread use of PET imaging of integrin expression is expected in the near future. In the next step, large clinical trials on the value of PET imaging of integrin expression for patient selection and monitoring in the context of antiangiogenic or combined cytotoxic / antiangiogenic therapy will be necessary to define its ultimate potential clinical value. In this respect, great promise lies in combined MR-PET imaging, which one day might help to assess functional and molecular parameters of angiogenesis in a one-stop-shop examination (Fig. 3). First prototypes for brain imaging have already been successfully implemented, and sequential as well as fully integrated whole-body clinical MR-PET scanners have recently been installed [53]. By combined MR-PET imaging, a complete assessment of the different aspects of tumour biology will hopefully become a reality and will be implemented in therapy planning and response evaluation as part of the concept of “personalized medicine”.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31
2. Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671-4
3. Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171-5
4. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-42
5. Galbraith SM. Antivascular cancer treatments: imaging biomarkers in pharmaceutical drug development. Br J Radiol. 2003;76:S83-6
6. Pio BS, Park CK, Pietras R, Hsueh WA, Satyamurthy N, Pegram MD. et al. Usefulness of 3'-[F-18]fluoro-3'-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol. 2006;8:36-42
7. Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE. et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058-65
8. Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293:855-62
9. Fani M, Andre JP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging. 2008;3:67-77
10. Matsumoto K, Kitamura K, Mizuta T, Tanaka K, Yamamoto S, Sakamoto S. et al. Performance characteristics of a new 3-dimensional continuous-emission and spiral-transmission high-sensitivity and high-resolution PET camera evaluated with the NEMA NU 2-2001 standard. J Nucl Med. 2006;47:83-90
11. Chatziioannou AF. Instrumentation for molecular imaging in preclinical research: Micro-PET and Micro-SPECT. Proc Am Thorac Soc. 2005;2:533-6
12. Atkin G, Taylor NJ, Daley FM, Stirling JJ, Richman P, Glynne-Jones R. et al. Dynamic contrast-enhanced magnetic resonance imaging is a poor measure of rectal cancer angiogenesis. Br J Surg. 2006;93:992-1000
13. Jeswani T, Padhani AR. Imaging tumour angiogenesis. Cancer Imaging. 2005;5:131-8
14. Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M. et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005;2:e70
15. Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491-7
16. Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew Chem Int Ed Engl. 1997;36:1374-89
17. Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H. et al. Radiolabeled αvβ3 integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med. 1999;40:1061-71
18. Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL. et al. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781-5
19. Haubner R. αvβ3-integrin imaging: a new approach to characterise angiogenesis? Eur J Nucl Med Mol Imaging. 2006;33(Suppl 1):54-63
20. Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet. 2001;40:539-51
21. Chen X, Park R, Shahinian AH, Bading JR, Conti PS. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl Med Biol. 2004;31:11-9
22. Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester HJ. et al. [18F]Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem. 2004;15:61-9
23. Haubner R, Wester HJ, Burkhart F, Senekowitsch-Schmidtke R, Weber W, Goodman SL. et al. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326-36
24. Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992;89:2018-22
25. Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808-12
26. Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc. 2004;126:5730-9
27. Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. Micro-PET imaging of αvβ3-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging. 2004;3:96-104
28. Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS. et al. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J Nucl Med. 2006;47:113-21
29. Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I. et al. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor αvβ3-integrin expression. J Nucl Med. 2004;45:1776-83
30. Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR. et al. MicroPET imaging of breast cancer alphav-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imaging Biol. 2004;6:350-9
31. Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem. 2004;15:41-9
32. Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S. et al. microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707-18
33. Li ZB, Cai W, Cao Q, Chen K, Wu Z, He L. et al. 64Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor αvβ3 integrin expression. J Nucl Med. 2007;48:1162-71
34. Poethko T, Schottelius M, Thumshirn G, Herz M, Haubner R, Henriksen G. et al. Chemoselective pre-conjugate radiohalogenation of unprotected mono- and multimeric peptides via oxime formation. Radiochimica Acta. 2004;92:317-27
35. Liu S, Liu Z, Chen K, Yan Y, Watzlowik P, Wester HJ. et al. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol Imaging Biol. 2010;12:530-8
36. Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48:1862-70
37. Balasubramanian K, Burghard M. Chemically functionalized carbon nanotubes. Small. 2005;1:180-92
38. Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev. 2006;58:1460-70
39. Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X. et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47-52
40. Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ. et al. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333-41
41. Beer AJ, Haubner R, Wolf I, Goebel M, Luderschmidt S, Niemeyer M. et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging αvβ3 expression. J Nucl Med. 2006;47:763-9
42. Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL. et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin αvβ3 expression in man. Clin Cancer Res. 2006;12:3942-9
43. Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I. et al. [18F]galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610-6
44. Schnell O, Krebs B, Carlsen J, Miederer I, Goetz C, Goldbrunner RH. et al. Imaging of integrin αvβ3 expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 2009;11:861-70
45. Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ. et al. Comparison of integrin αvβ3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49:22-9
46. Metz S, Ganter C, Lorenzen S, van Marwick S, Herrmann K, Lordick F. et al. Phenotyping of tumor biology in patients by multimodality multiparametric imaging: relationship of microcirculation, αvβ3 expression, and glucose metabolism. J Nucl Med. 2010;51:1691-8
47. Bach-Gansmo T, Danielsson R, Saracco A, Wilczek B, Bogsrud TV, Fangberget A. et al. Integrin receptor imaging of breast cancer: a proof-of-concept study to evaluate 99mTc-NC100692. J Nucl Med. 2006;47:1434-9
48. Morrison MS, Ricketts SA, Barnett J, Cuthbertson A, Tessier J, Wedge SR. Use of a novel Arg-Gly-Asp radioligand, 18F-AH111585, to determine changes in tumor vascularity after antitumor therapy. J Nucl Med. 2009;50:116-22
49. McParland BJ, Miller MP, Spinks TJ, Kenny LM, Osman S, Khela MK. et al. The biodistribution and radiation dosimetry of the Arg-Gly-Asp peptide 18F-AH111585 in healthy volunteers. J Nucl Med. 2008;49:1664-7
50. Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ. et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879-86
51. Winick J. A Proof-of-Concept Study to Assess the Ability of [18F]AH-111585 PET Imaging to Detect Tumours and Angiogenesis; ClinicalTrials.gov; November 28, 2007 ed. US: National Institutes of Health. 2007
52. Gaertner FC, Schwaiger M, Beer AJ. Molecular imaging of αvβ3 expression in cancer patients. Q J Nucl Med Mol Imaging. 2010;54:309-26
53. Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M. et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459-65
54. Aumailley M, Gurrath G, Müller G, Calvete J, Timpl R, Kessler H. Arg-Gly-Asp constrained within cyclic pentapeptides: strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991;291:50-4
55. Schottelius M, Laufer B, Kessler H, Wester HJ. Ligands for mapping alphavbeta3-integrin expression in vivo. Acc Chem Res. 2009;42:969-80
56. Pichler B, Braumueller H, Haubner R, Sakrauski AK, Kneilling M, Senekowitsch-Schmidtke R. et al. Monitoring of cellular immunotherapy in RIP1-Tag2 transgenic mice with radiolabelled RGD-peptides. J Nucl Med. 2002;43:122P
57. Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric Cyclic RGD Peptides as Potential Tools for Tumor Targeting: Solid-Phase Peptide Synthesis and Chemoselective Oxime Ligation. Chem Eur J. 2003;9:2717-25
58. Wester HJ, Kessler H. Molecular Targeting with Peptides or Peptide-Polymer Conjugates: Just a Question of Size? J Nucl Med. 2005;46:1940-5
Author contact
![]() Corresponding author: Dr. Ambros Beer, Department of Nuclear Medicine, Klinikum rechts der Isar, Ismaningerstr. 22, 81675 Munich, Germany, Tel.: +49 89 4140 6085; E-mail: ambros.beerde
Corresponding author: Dr. Ambros Beer, Department of Nuclear Medicine, Klinikum rechts der Isar, Ismaningerstr. 22, 81675 Munich, Germany, Tel.: +49 89 4140 6085; E-mail: ambros.beerde
 Global reach, higher impact
Global reach, higher impact